Prof. Dr. Esther Schnettler
Telefon: +49 40 285380-208
E-Mail: schnettler@bnitm.de
Research Group Leader
Telefon: +49 40 285380-208
E-Mail: schnettler@bnitm.de

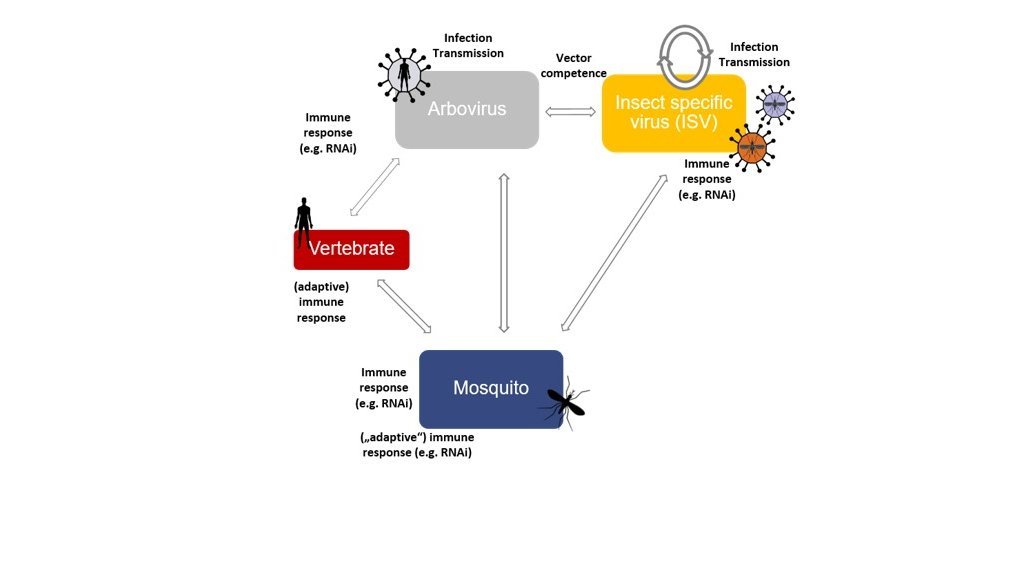
My group works on arthropod-borne viruses (Arboviruses) that are transmitted by biting arthropods (e.g. mosquitoes, ticks and midges) to mammals (including humans) and can cause severe disease. The main focus is on mosquito-transmitted viruses, but we also have projects on tick and midges transmitted viruses.
The research focus of my group is the study of the complex arbovirus-host-vector interactions, specifically the (molecular) mechanisms that control vector competence and the factors that affect arbovirus infection in vertebrates. This knowledge is used to develop new methods for vector control and thereby reduce the transmission of these viruses to humans and animals.
Prof. Dr. Esther Schnettler
Telefon: +49 40 285380-208
E-Mail: schnettler@bnitm.de





Wir verwenden Cookies, um unseren Besuchern ein optimales Benutzererlebnis zu ermöglichen.
Datenschutzerklärung
So verstehen wir besser, wie Besucher unsere Webseite nutzen.
Damit wir online Karten von Google oder Baidu anzeigen können.
Wir benötigen diese, damit diese Seite einwandfrei funktioniert.
