Research projects
The projects in this junior research group focus on deepening the understanding of the mechanisms that renders some species and not others susceptible to the infection of emerging viruses such as NiV, EBOV and MARV. A second focus consists of exploring the bat virome and providing a bridge between ecology, virus discovery and pathogenesis. Altogether, our research aims to provide vital intelligence to predict and reduce the risk of future emergence of zoonotic viral diseases and characterize the potential risk for public health of viruses discovered in field studies.
Use of xenochimeric mice to study emerging viruses in different hosts
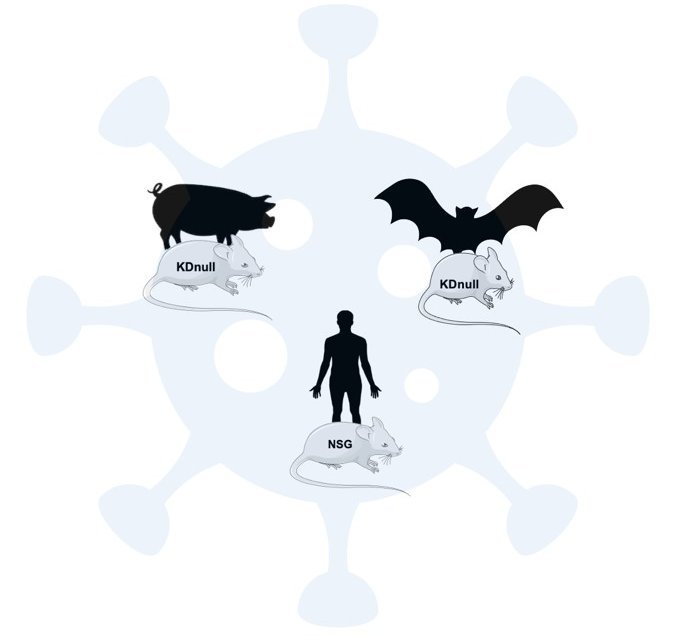
Despite the importance for global public health of zoonotic viruses, very little is known about their pathophysiology. There are often many differences in the symptoms between the natural, transmission and human hosts. Zoonotic infections often appear as asymptomatic and non-lethal in the natural reservoir host (frequently bats and rodents), but may induce severe and potentially lethal disease in humans. This is the case for Nipah virus (NiV), Ebola virus (EBOV) and Marburg virus (MARV). To date there is no animal model that would allow a good comparison between hosts for these viruses. Therefore, currently there is no possibility to experimentally determine the pathogenic potential of viruses and understand the mechanisms that render some species susceptible to the infection.
Our team has developed xenochimeric NSG mice (NOD scid gamma mouse) transplanted with species-specific hematopioietic progenitor cells thus allowing the reconstitution of the species-specific hematopoietic compartment generating a functional species-specific immune system in vivo. This model allows (i) to study the pathophysiology of not fully characterised or newly discovered emerging viruses, (ii) to correlate species-specific susceptibility factors and (iii) to provide an in vivo platform to test therapeutic and preventive strategies as medical countermeasures against emerging infections.
Study of bats virome
Bats are abundant, diverse, and geographically widespread. These mammals are suitable hosts of viruses and other disease agents. It is clear though that we do not know enough about bats’ ecology and very little is done to identify the distribution of the species and which ones are more prone to have contact with humans. Active surveillance is both urgent and essential to predict and mitigate the emergence of zoonotic viruses. We have limited knowledge of the viral population and ecological diversity harbored by wildlife and this, complicates the study of emerging infection diseases (EIDs).
Our team focuses in exploring the epidemiology of reservoir host-zoonotic virus maintenance in bats and provide answers to the role of bats in disease emergence. We intend to understand the prevalence, the geographical distribution and the genetic diversity of pathogens in bats thus providing a valuable information to prevent and control EIDs.


Capacity building
Emergent diseases and their subsequent spread cause a significant impact on global health and economy. An early detection of emerging pathogens could prevent future pandemics. However, the limited means to perform this early detection in hotspots such as the rainforest area of Africa challenge this purpose. Through a multifaceted approach, our team’s purpose is to collaborate with African local teams to identify the factors that need to be improved in order to increase research output in Africa as well as the diagnostic capacity and cutting-edge research in the country.
We have established diagnostic capacity for several emerging virus families in Republic of Congo and performed surveillance studies in both humans and wildlife. We have improved and strengthened our collaboration with our African partners by establishing protocols, teaching laboratory practice routines for diagnostics, and performing research studies. Moreover, we have trained African local personnel in order to perform several molecular and immunology biology techniques.
The purpose of our team is to improve the capacities and preparedness of emerging diseases of African local laboratories by rapidly detecting severe endemic outbreaks.











