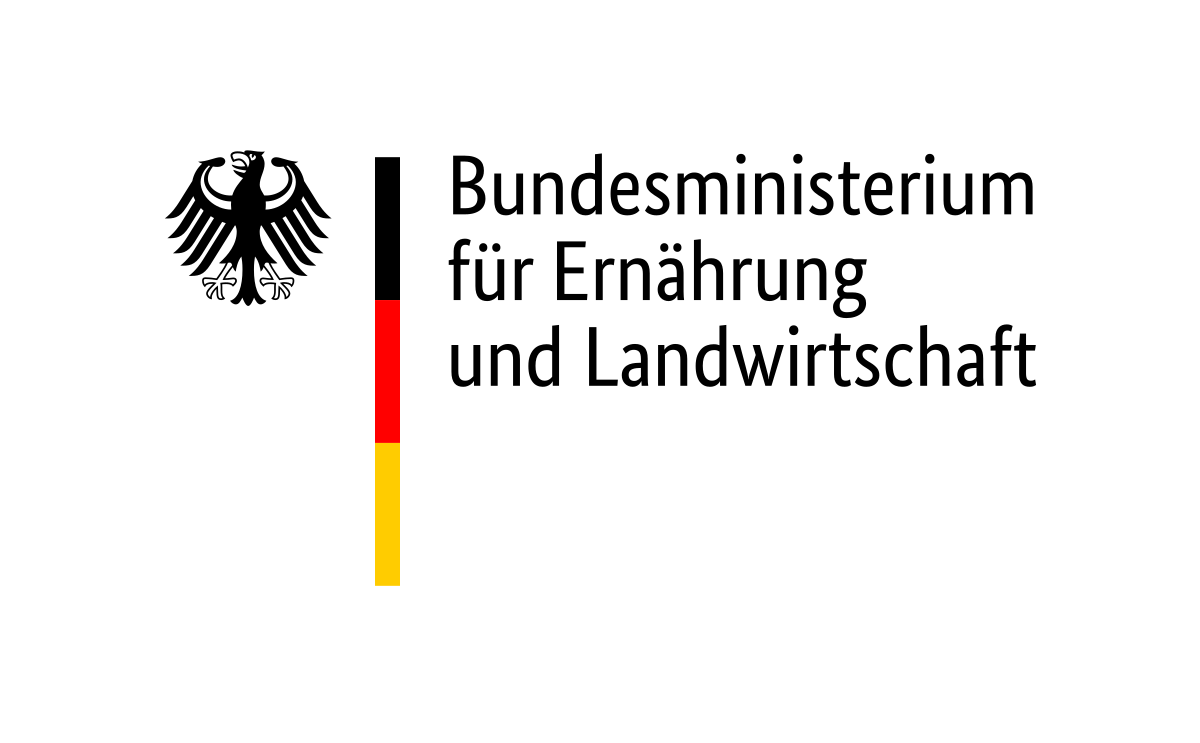
Arbovirus-mosquito interaction
Mosquitoes acquire and transmit arboviruses by blood-feeding on susceptible hosts. Following infection, the virus actively replicates and disseminates, crossing various tissue/cell barriers within the mosquito. After reaching and replicating in salivary glands and getting excreted into the saliva, the virus can be transmitted to a susceptible host during the next blood meal. The mosquito vector actively fights the virus infection, but is often not successful to clear it. Thus, to ensure transmission to a new host, the virus needs to overcome mosquito immunity in different tissues. While mosquitoes have to counteract virus infection to keep virus replication in control. This arms race is thought to strike a balance between successful viral replication and survival of the mosquito vector; leading to non-pathogenic persistent infections in mosquitoes. Besides, the arbovirus needs to interact with a variety of mosquito proteins to ensure successful replication and virus production. This is similar to arbovirus infections in the vertebrate hosts; however in contrast to infection in mosquitoes, vertebrate infections normally lead to cytophaticity, apoptosis of infected cells, disease and often death of the infected host.
At the moment most of our research is done in Aedes aegypti derived cells and mosquitoes; however we also perform experiments with other important mosquito vectors, like different Culex species.
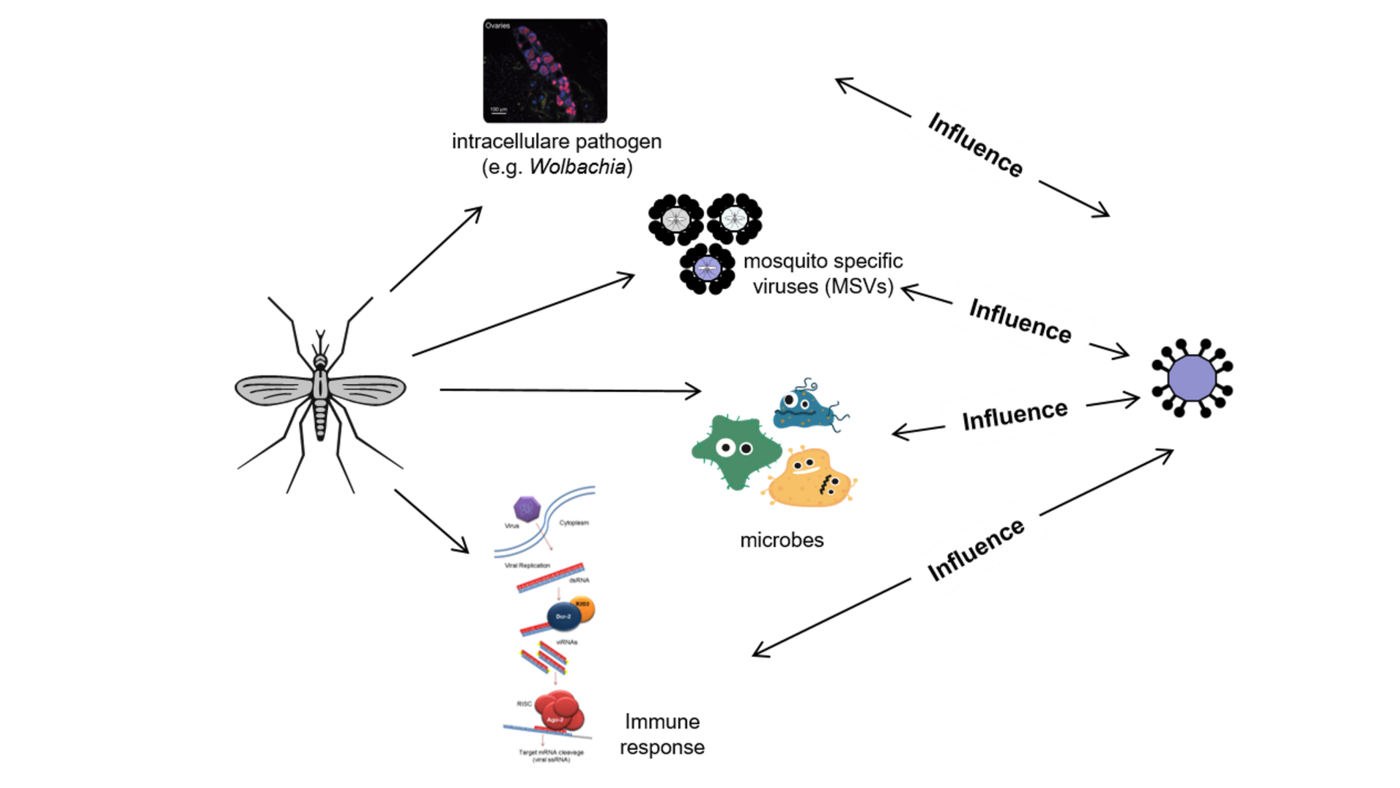
Immune response in mosquitoes, RNA interference (RNAi)
The RNA interference (RNAi) pathway, the major antiviral response in mosquitoes and other arthropods, degrades viral RNA, with the use of small RNA molecules, and thereby inhibits virus replication. RNAi is categorised into three pathways depending on the size of the small RNAs and the involved key proteins, with two of them shown or suggested to act antiviral: siRNA with Argonaute 2 (Ago2) and Dicer 2 (Dcr2), piRNA with Piwis and Ago3.
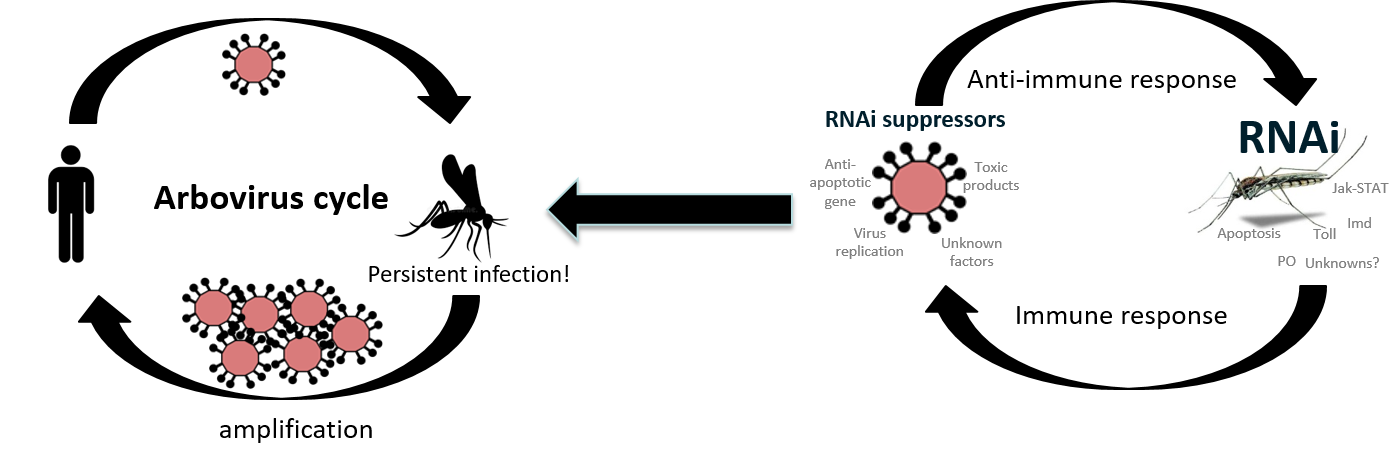
In addition, the presence of viral sequences in the mosquito genome and production of the corresponding virus-specific small RNAs entering the antiviral RNA interference pathway have been proposed to act as an “adaptive immune response”. This shapes vector competence even in the same mosquito species depending on previous virus exposure and the ability to be passed on to offspring.
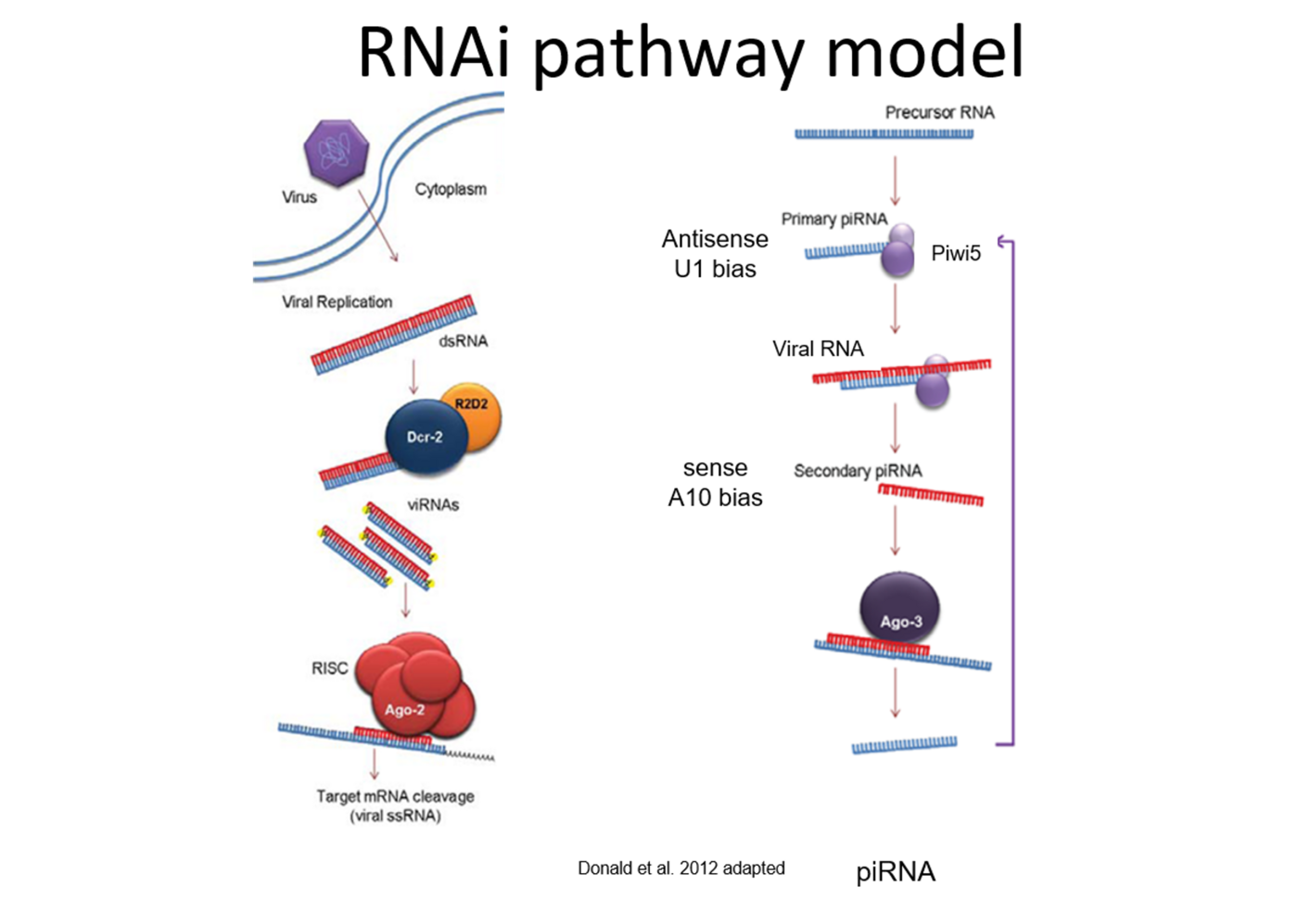
Present research in the group is looking at the antiviral RNAi response in mosquitoes against different arboviruses to determine which response is generally antiviral and which proteins, factors or pathways are virus (family) specific.
Results
Using a combination of mosquito-borne and midge-borne viruses, we have shown that the RNAi response differs for the same virus in vector versus non-vector cells (Dietrich et al.2017); linking the RNAi response to vector competence. In addition, a mosquito protein (Spindle-E) was identified that only acts antiviral against alphaviruses, like CHIKV (Varjak et al., Viruses 2018). Proteins that regulate the antiviral response against different arboviruses in mosquitoes have been identified and characterized (Varjak et al. 2020 Viruses; McFarlane et al. 2020 mSphere). This includes detailed investigation of the interacting proteins and RNA molecules with the “non-canonical” Piwi4 protein of Aedes aegypti, to understand its broad antiviral activity against all tested arboviruses.
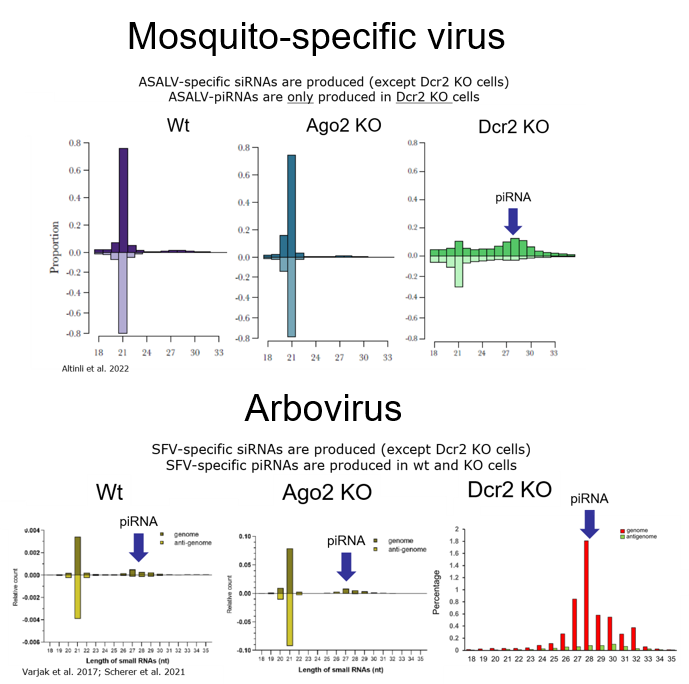
Detailed investigation with different arboviruses either during single or co-infection in mosquitoes and derived cells, showed that all tested arboviruses induce the antiviral RNAi response, represented by the production of virus specific small RNAs. However, the importance of certain RNAi-related proteins during the antiviral response can differ depending on the investigated virus, infection phase or co-versus single infection. Specifically, we found that Ago2 (a key protein of the siRNA pathway) does not act antiviral against Zika virus (ZIKV) and dengue virus (DENV1), which is in contrast to all other so far tested arboviruses (Varjak et al. 2017; Scherer et al. 2021; Leggewie et al. Plos NtD accepted).
Moreover, comparison of the antiviral RNAi response between an arbovirus and a mosquito-specific viruses, belonging to the same virus family, highlighted common and conserved responses and proteins as well as some interesting differences (Altinli et al. 2021).
Detailed mutational analysis of Ae. aegypti Dicer-2 protein (key protein in the antiviral siRNA pathway) has resulted in a model for viral siRNA production and linked this data to the antiviral activity of Dcr-2 against arboviruses. Moreover, functionally relevant amino acids were found to be conserved in haplotype Dcr2 sequences from field-derived Ae. aegypti across different continents (Gestuveo et al. 2022).
Most work investigates the interaction between arboviruses and mosquitoes, uses Ae. aegypti mosquitoes or derived cells. However, information about the antiviral response in another important mosquito vector (e.g. for West nile virus): Culex mosquitoes, and its importance for vector competence is sparse or even lacking. Experiments comparing the antiviral response and identifying the antiviral proteins in Culex mosquitoes, highlighted common but also important differences between these mosquitoes (Altinli, Leggewie et al. 2023).
RNAi-based adaptive immunity in Aedes aegypti against arboviruses (DFG funded)
Mosquitoes transmit pathogenic arthropod-borne (arbo)viruses, like Dengue, Chikungunya and Zika viruses, causing outbreaks in different parts of the world. It is hypothesized that the efficiency of virus transmission (vector competence) of a mosquito, dependents on its antiviral response. Mosquitoes (even of the same species) has been shown to differ in their antiviral response and thereby vector competence.
The project investigates the hypothesis of an inheritable adaptive antiviral response in Ae. aegypti, that primes mosquitoes and thereby shapes the vector competence of mosquitoes (ability to transmit virus infection). This adaptive antiviral immune response in mosquitoes result from previous encounters with viruses and depends on the production of viral DNA and the RNAi machinery. For this, classical and molecular virology methods in combination with established knock out cell lines, silencing and next generation sequencing are employed. This combinational approach will enable us to investigate the hypothesis of an inheritable adaptive RNAi-dependent antiviral immune system in mosquitoes in detail and to identify differences as well as common features in this response for the main arbovirus families. Understanding the ability of mosquitoes to acquire and develop viral tolerance, specifically for arboviruses, will provide important insights into factors affecting viral infection and transmission by mosquitoes (vector competence). This knowledge may serve as promising targets for developing preventive approaches to control arbovirus outbreaks
Vector competence
To determine vector competence of mosquitoes, it is important to be able to identify how many mosquitoes get infected (infection rate) and how many of the infected mosquitoes have virus in their saliva (transmission rate). Especially for the last one, specific methodology is needed to collect saliva of infected mosquitoes for further analysis. In collaboration (Prof. Schmidt-Chanasit, Prof. Tannich; BNITM), the methodology of the forced saliva assay was established under high security (BSL3) insectary conditions (Heitmann et al., J Vis Exp. 2018). Using this methodology, vector competence for several viruses was determined in different mosquito species.

Results
In collaboration with the group of Prof. Schmidt-Chanasit, it was shown that Aedes albopictus, recently established in Germany, is competent at temperate climates realistic for Germany to transmit CHIKV (Heitmann et al., Euro Surveil 2018). Moreover, the indigenous and abundant Culex torrentium mosquito has a high vector competence for West-Nile virus (Jansen et al., Viruses 2019) and that the competence is linked to temperature. Mostly, lower incubation temperatures result in less mosquitoes showing virus in their saliva and thereby having a reduced vector competence.
Vector competence of relevant mosquitoes for viruses circulating in Germany and candidates for global outbreaks (DZIF)
Mosquito protein-virus interaction and importance
As arboviruses, generally only encode for a small number of proteins, they are strongly depended on host or vector proteins in the infected cells to ensure successful virus replication and virus production. Interaction and importance of arboviral proteins with host proteins (e.g. humans) have been studied for a long time and a variety of proteins and their function and importance for the arbovirus infection is known. However in case of mosquito vector proteins and their importance and interaction with arboviral proteins is little to none known.
Identification and characterization of NS5-interacting factors involved in anti-flaviviral response in Aedes mosquito (collaborative project with Dr. Sarin Chimnaronk, Mahidol University Thailand; DFG-NSTDA)
Mosquito-borne flaviviruses include many of the most prevalent viral scourges known to humanity, such as dengue virus (DENV). DENV exists as four closely related but antigenically distinct serotypes (DENV-1, -2, -3, and -4), all of which have Aedes aegypti mosquitoes as their primary vector. Like DENV, ZIKV is also mainly transmitted by Ae. aegypti and is often endemic in the same area than DENV. Field studies and laboratory infections clearly showed that Ae. aegypti mosquito is highly permissive to coinfection with DENV and ZIKV.
While DENV and ZIKV infections are often asymptomatic or elicit mild flu-like symptoms; some infected people develop strong pathogenicity, like severe bleeding, organ impairment and/or plasma leakage in case of DENV or birth defects, known as congenital Zika syndrome, and even death of infected fetus in case of ZIKV. Despite this great burden, vaccines and specific antivirals remain elusive.
Very little is known at the moment about the interaction and importance of mosquito proteins during DENV or ZIKV infection (either single or co-infection).

The overall aim of the collaborative project is to identify mosquito proteins interacting with DENV-2 NS5 protein, for the first time, during natural virus infection, their importance for DENV-2 in single or coinfection with ZIKV and the extrapolation to other DENV serotypes. Therefore, we use a recently created DENV expressing a tagged version of NS5, which is essential due to the lack of antibodies. Understanding the interaction of DENV NS5 and mosquito proteins and its importance for successful infection of the vector could identify valuable targets for novel intervention strategies. Besides it could give information about factors affecting vector competence (ability of a mosquito to successfully transmit a certain virus) in single and coinfection and thereby helps to predict future outbreaks.