Projects
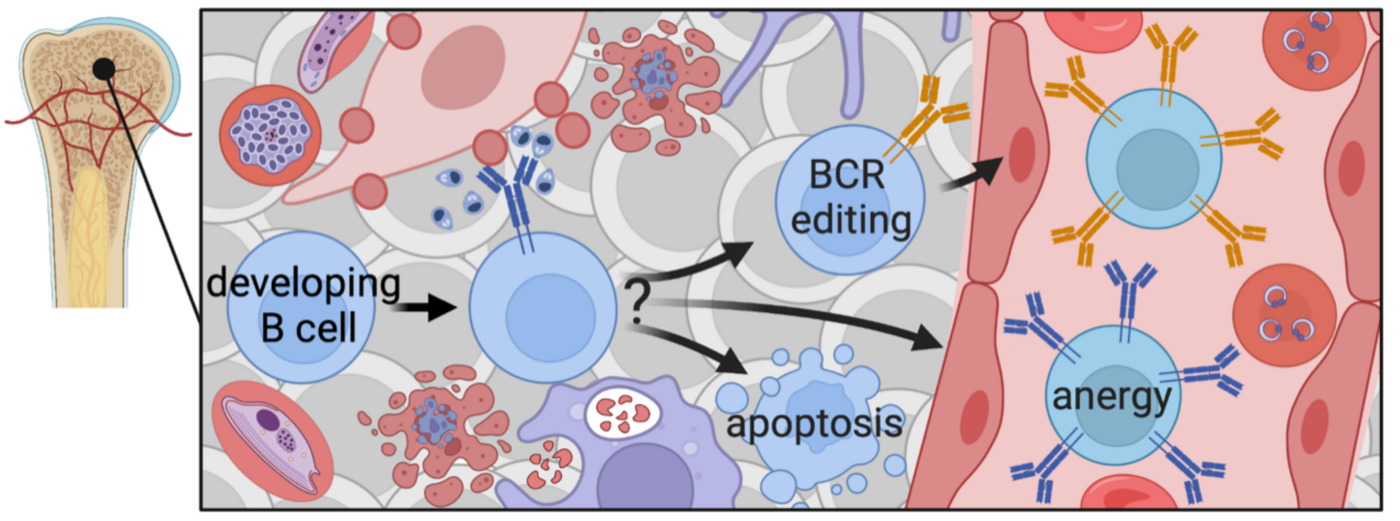
Plasmodium infection-induced perturbation of the bone marrow B cell compartment and its impact on malaria immunity
(LCI Graduate School Project, Aws Aljnabi)
Natural immunity to malaria develops slowly and malaria vaccine trials have been disappointing, with low efficacy and poor longevity, particularly in areas with high malaria exposure. Many pathogens (Malaria, Leishmania, Trypanosoma, Mycobacterium tuberculosis (Mtb) and HIV) can persist in the human and murine bone marrow (BM), where B lymphocytes develop. As they mature, B cells that bind to molecules present in this environment undergo negative selection and are deleted from the repertoire to prevent autoimmunity. Our research investigates the hypothesis that through persistence in the BM, pathogens can exploit this endogenous mechanism for self-tolerance. This would lead to pathogen-specific tolerance and could contribute to inefficient acquisition of natural and vaccine-induced immunity. Using human samples from a malaria-endemic region in Mali, as well as a chronic malaria mouse model, experimental approaches involve cellular immunology, immunofluorescence analysis and next-generation sequencing of the B cell receptor (BCR) repertoire. It will be elucidated whether pathogen persistence in the BM can undermine efficient acquisition of immunity and illuminate underlying mechanisms, that will ultimately prove critical for the development of an effective vaccine against pathogens such as malaria, HIV and Mtb.
Investigating immune regulatory mechanisms arising from chronic malaria exposure that protect from autoimmune pathology
The interplay between infections and autoimmune disorders has been the subject of comprehensive exploration, mainly focusing on the development of pathologic autoimmunity following infection. In an ongoing longitudinal study in malaria-endemic Mali (n=602), we found a high prevalence of autoantibodies in a cohort of children and adults with no history of autoimmune diseases. When we prospectively assessed the relationship between baseline autoantibody levels in healthy individuals and their risk of clinical illness during a subsequent infection, we found that high levels of autoantibodies before the 6-month malaria season independently predicted reduced risk of acute febrile malaria caused by Plasmodium falciparum infection during the ensuing malaria transmission season. Despite the high prevalence of circulating autoantibodies we and others have observed in these populations, and although genetic susceptibility to autoimmune disease is higher among Africans outside of malaria-endemic regions, autoimmune diseases appear to be rare among those residing in endemic regions of Africa. Future work will investigate how immune regulatory mechanisms, arising from chronic malaria exposure, can shield individuals with high levels of autoantibodies from developing autoimmune disease.
Longitudinal maturation and diversification of the B cell receptor repertoires over years of repeated malaria infections
(DZIF MD-fellowship, Helen George)
V(D)J recombination creates hundreds of billions of antibodies (B cell receptors, BCRs) that collectively provide broad protection against a vast diversity of pathogens. The antibody repertoire is further diversified through somatic hypermutation (SHM). Quantification of this diversity has been possible since the recent development of high-throughput immune repertoire sequencing. Many studies have examined how the BCR repertoires change in response to vaccination or infection,yet few have explored the longitudinal evolution and dynamics of the immune repertoire in healthy individuals. Using a unique set of samples obtained from an ongoing longitudinal cohort study in malaria-endemic Mali, we are characterizing the BCR repertoires as they evolve over the course of many years, assessing the impact of successive acute malaria episodes and generating key insights into the longevity of malaria-specific B cell clones.
The cohort studies in Mali are made possible through a close collaboration with the laboratory of Peter Crompton, NIAID/NIH, as well as an experienced team of clinicians and scientists at the Malaria Research and Training Center at the University of Bamako, and support of the Mali study by the NIAID International Centers for Excellence in Research (ICER) program.









