Our research projects
The influence of sex hormones on monocyte immune responses in transgender individuals
Barbara Honecker, Claudia Marggraff
Many parasitic, bacterial, or viral infections manifest with distinct sex biases in disease prevalence or severity. Differing monocyte immune responses between male and female individuals have been linked to differing disease severity in some of these infections, for example in hepatic amebiasis. Previous studies from our and other groups have shown that sex hormones mediate these immune responses. For example, testosterone was found to enhance a pro-inflammatory phenotype in monocytes. In this project, we aim to understand the effect of sex hormones on monocytes in further detail. To this end, we use PBMCs from transgender men and women before and during gender-affirming hormone therapy (GAHT) and expose them to infectious stimuli in vitro. By employing methods such as single-cell RNA sequencing and spectral flow cytometry, we are able to characterize detailed immune responses occurring under the influence of testosterone or estrogen treatment.
This project is a part of the Research Unit 5068 Sex Differences in Immunity.
Funding:
Sex-specific modulation of immune responses in myeloid cells by the metabolite itaconate
Barbara Honecker, Piet Paulsen, Helena Fehling, Lara Buer
In response to infectious stimuli such as bacterial LPS, the metabolite itaconate is synthesized in myeloid cells by the enzyme aconitate decarboxylase 1 (ACOD1) using cis-aconitate of the mitochondrial TCA cycle. Itaconate exhibits many anti-inflammatory and anti-oxidative properties and has been shown to restrict intracellular bacterial growth and viral replication in several studies, resulting in promising therapeutic potential. In this project, we assess whether sex differences in infectious diseases, in which immune responses are modulated by itaconate, can be linked to differences in the activity of the immune metabolism in monocytes and macrophages from cisgender men and women.
Furthermore, we strive to elucidate the anti-parasitic and anti-bacterial potential of itaconate and its derivatives in Leishmania infantum-infected macrophages (performed at BNITM) as well as Mycobacterium tuberculosis-infected macrophages (performed at RC Borstel in cooperation with the group of Dr. Bianca Schneider). To assess the impact of metabolite treatment on macrophage infection, we use high-content screening confocal microscopy. Both leishmaniasis and tuberculosis exhibit male-biased sex differences. Therefore, by using monocyte-derived macrophages from men and women, our study aims to provide novel insight into mechanisms underlying sex differences in intracellular parasite/bacterial control and disease manifestation, as well as potential therapeutic strategies.
This project is a part of the Research Unit 5068 Sex Differences in Immunity and performed in collaboration with Prof. Dr. Frank Pessler (TWINCORE Hannover, HCI Braunschweig).
Funding:
Organoid-based infection model for the analysis of interorganic immune responses
Svenja Stenzel, Helena Fehling, Danyel Rahimi
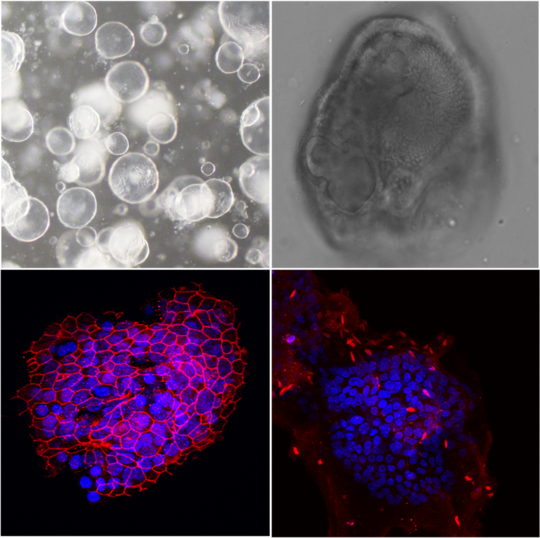
Organoids are three-dimensional cell structures derived from stem cells and grown in vitro. They partially replicate the architecture and function of selected organs and can be used to study inter-organ communication in response to infections with different pathogens, such as influenza virus, in a human-relevant context.
In this project, human pluripotent stem cells (iPSCs) are differentiated into functional lung and liver organoids by applying defined culture protocols that mimic key developmental signals through the use of stage-specific growth factors, extracellular matrix components and controlled environmental conditions. These organoids will be introduced into a so-called multi-organ chip system - a platform system in which the organoids are permanently connected via microfluidic channels. This allows the communication between the organoids via soluble mediators, released in response to infection. The primary aim of this model is to infect the lung organoid with influenza viruses in order to trigger a local immune response and to investigate how this response affects the liver organoid via the chip’s common circulatory system.
Funding: Leibniz Center for Infection Graduate School
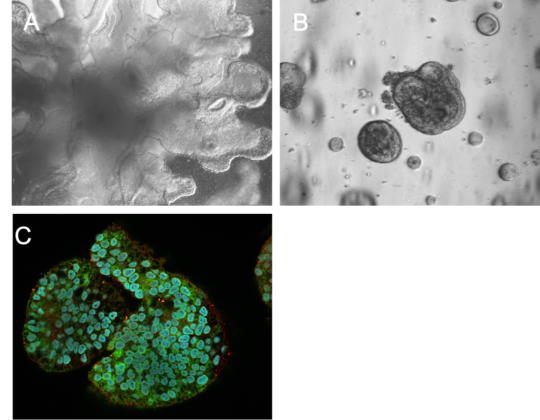
A) Lung organoid B) Hepatic organoid C) Hepatic organoid expressing alpha-1-Antitrypsin (hepatocyte marker) © BNITM | Svenja Stenzel
Sex-specific responses of monocytes to infectious challenges
Charlotte Hansen, Nele Wichern
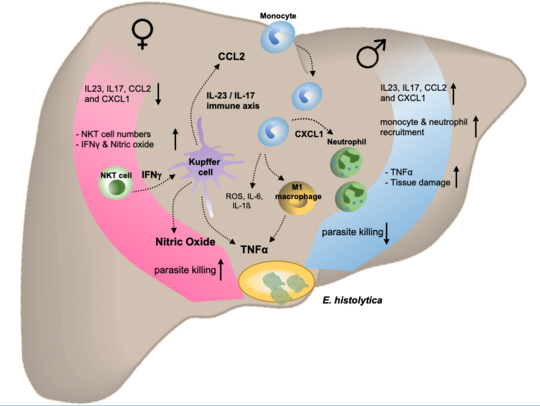
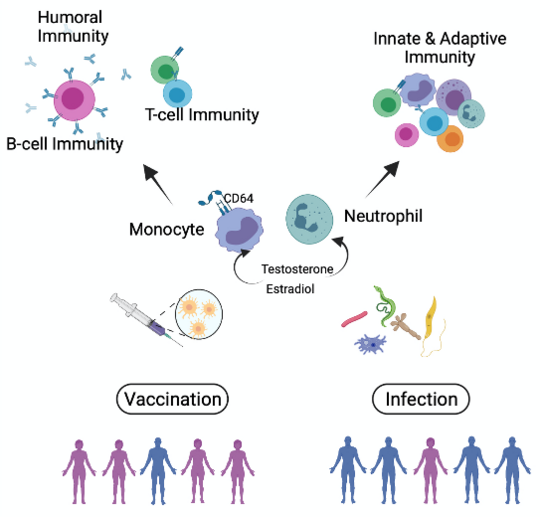
Monocytes play an important role in immune control of infections and in the success of vaccination. Like other cells of the immune system, monocytes are influenced by biological, sex-specific factors. For example, many parasitic diseases such as leishmaniasis and amebiasis occur more frequently in males. The mouse model for hepatic amebiasis mimics the sex difference as observed in humans and substitution of females with testosterone abolished their resistance. As key mediators of inflammation, monocytes must be tightly regulated, but when this is not the case, as in amebiasis, excessive activation can result in immunopathology and destruction of host tissue. Our previous investigations revealed that TNFα and recruitment of classical, inflammatory monocytes and neutrophils substantially contribute to liver damage during parasitic infection. The underlying immune cascade involves the IL-23/IL-17 immune axis and monocyte/neutrophil-attracting cytokines like chemokine ligand (CCL) 2 and the C-X-C motif ligand 1 (CXCL1). Intriguingly, TNFα and both chemokines are higher in male compared to female mice and partially also in humans upon infectious challenge. The production of these proinflammatory cytokines is further increased by treatment with the most active androgen, dihydrotestosterone. On the other hand, the prerequisites to recognize a foreign antigen are stronger in monocytes of female individuals. This also leads, for example, to a stronger vaccination response in women than in men. Among other mechanisms, certain Fcgamma receptors on the surface of monocytes are responsible for this.
We hypothesize that sex hormones substantially modulate monocyte functions and aim to investigate essentially underlying androgen-dependent signaling pathways
The immune response to foreign agents differs between women and men.
We will study the influence of sex hormones on cells of the innate immune system and the impact on the efficacy of vaccination and the outcome of parasitic infection.
Funding:
Forschergruppe 5068; "Sexdifference in Immunity" (Deutsche Forschungsgemeinschaft)
Influence of biological sex on the infection of human macrophages with Leishmania parasites and response to immunostimulatory therapy
Dr. Helena Fehling, Annika Bea
Many parasitic diseases occur with a sex-bias in terms of incidence, morbidity and mortality towards male individuals. Leishmaniasis is such an example as significantly higher number of cases are present in male patients in various endemic areas. However, this phenomenon cannot only be explained by cultural or behavioral determinants, indicating that biological factors can alter the susceptibility towards an infection and influence the outcome of the disease. Besides sex steroid hormones, such as testosterone or estradiol, chromosomal factors (XX, XY) can affect the host immune response and potentially the pathogenic agent respectively. Leishmania parasites can survive and multiply in innate immune cells, such as macrophages or dendritic cells, while suppressing an effective clearance of the parasite by creating an anti-inflammatory environment. Macrophages are the primary host cells and the key players to determine the outcome of the infection. To understand biological sex differences in leishmaniasis, we investigate the influence of the host sex in the infection of macrophages by Leishmania ssp. on a cellular, molecular biological and immunological level.
Since intracellular microorganisms such as Leishmania parasites or bacteria of the genus Mycobacterium that target immune cells can circumvent their clearance by manipulating the innate and the acquired immune response of the host, immune stimulatory molecules that activate or re-activate target cells such as macrophages represent promising therapeutic tools for the treatment of these diseases.
We recently isolated such an immunostimulatory molecule from the membrane of Entamoeba (E.) histolytica, a lipopeptidephosphoglycan (EhLPPG). The native molecule exhibited considerable immune cell stimulation leading to the reduction of the intracellular load of leishmania parasites in vitro and in a mouse model for the disease. In cooperation with Prof. Yukari Fujimoto from the Keio University in Japan and Prof. Chris Meier from the University of Hamburg we developed a series of synthetic analogs deduced from the two Phosphatidylinositol anchors of EhLPPG. So far, several analogs reproduced the anti-leishmanial activity of the parent compound in vitro and in vivo, presumably by inducing production of pro-inflammatory cytokines, and exhibited bactericidal activity against Mtb in vitro (Patent BNI002).
Funding Annika Bea: https://www.joachim-herz-stiftung.de/
Former Projects
Organoids as host models for parasitic infections
Melanie Lütkemeyer
Parasitic infections of the liver are the subject of extensive research projects, but much of the knowledge gained is based on the use of mouse models. Advantages and limitations of these models have long been known. As an alternative, we use liver organoids for our research work.
These are in vitro cultures that enable the long-term cultivation of human liver cells in a 3D composite. In this way, both functional and structural properties of the liver can be recreated in miniature. The required liver cells can be obtained from the excess tissue of biopsies or tumour resections.
We use the liver organoids to model parasitic infections using human cells and to study the interaction with immune cells in detail in the form of
co-cultures.
Funding:
Extracellular vesicles as communicators between parasite and host immune system
Barbara Honecker
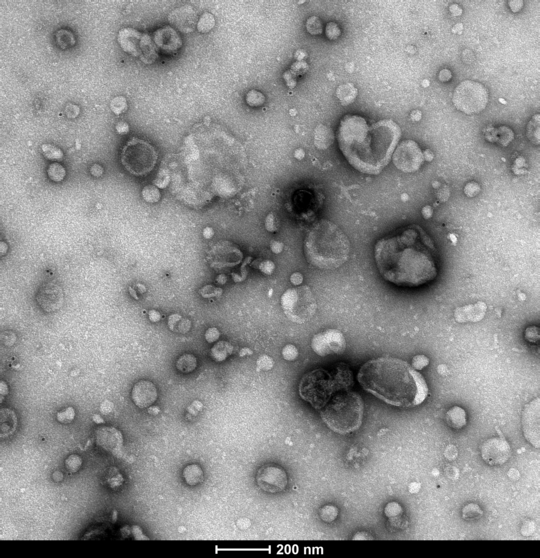
Extracellular vesicles (EVs) are small particles released by cells that contain protein or microRNA (miRNA), among other types of cargo. For many parasitic disease models, it is known that EVs can play a role in the interaction between parasite and host and contribute to immune responses.
In this project, we investigate EVs secreted by Entamoeba histolytica, causative agent of the disease amebiasis. EVs are analyzed with regard to their biological properties as well as their immune-stimulatory capacities. In order to determine to which extent EVs play a role in communication with the host immune system, EVs isolated from parasite cultures are used to stimulate monocytes and neutrophils in vitro. Both immune cell types are known to be involved in the immune pathology of amebiasis. Immune cells derived from both male and female mice are used in order to investigate putative sex differences in response to EV stimulation. Furthermore, two clonal parasitic cell lines differing in their pathogenicity are used in order to elucidate pathogenicity mechanisms of the parasite.
Funding: : https://www.joachim-herz-stiftung.de/
Immune processes underlying ALA formation and regeneration
Stefan Hoenow, Marie Groneberg, Claudia Marggraff
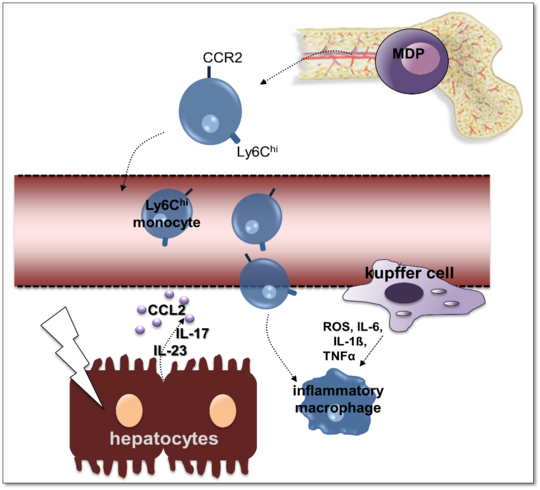
Human amebiasis results from intestinal infections with the protozoan parasite Entamoeba histolytica (E. histolytica). The two major clinical symptoms that derive from the infections, the amebic colitis and the amebic liver abscess (ALA), represent major health problems in subtropical and tropical areas as well as in travellers. The parasite usually asymptomatically colonizes the bowel system, but occasionally it invades the mucosa and spreads via the blood stream into other organs, mainly in the liver. To investigate immunological backgrounds for the development of ALA, we have established a mouse model that resembles the human disease in terms of a comparable pathology and a similar sex distribution. Using selective depletion strategies and various knockout mutant mice, we were able to show that innate immune cells like the inflammatory Ly6Chi monocytes, but also liver resident Kupffer cells substantially contribute to the liver destruction via production of TNFα. IL-23 further contributes to immunopathology of ALA by stabilizing IL-17 production and CCL2 expression. On the other hand, tissue repair is initiated by the presence of anti-inflammatory Ly6Clo monocytes in the liver that produce IL-13 and further develop into alternative activated M2 macrophages expressing Arginase 1.
Funding:
SFB841, " Leberentzündung: Infektion, Immunregulation und Konsequenzen" Project A2
Sex dimorphisms in the immune system
Julie Sellau, Marie Groneberg
An increasing body of evidence suggests an involvement of sex-based influences on the outcome of infectious, autoimmune and tumor diseases. This could be either attributed to X- or Y-chromosome linked-genes, environmental risk factors or sex hormones that act on immune responses involved in the outcome of a disease. Amebic liver abscess, a major severe symptom arising from human infections with the protozoan parasite Entamoeba (E.) histolytica, occurs independent from the infection rate with a clear sex bias towards adult men. With the aid of a mouse model that reflects the same sex dimorphism as observed in human, we found that testosterone significantly influenced the outcome of the disease. Female mice treated with testosterone lost their ability to control the abscess development and the parasite burden in the liver and Natural killer T cells, which were crucial for the control of the disease, produced less amounts of the protective cytokine IFNγ. In contrast, abscess development in male individuals was enabled by an immunopathology mediated by a C-C chemokine ligand (CCL) 2-dependent recruitment of inflammatory monocytes and the production of TNFα and Nitric Oxide. Interestingly, we found higher CCL2 serum levels in the serum of men and male mice infected with the parasite compared to female individuals. In a larger consortium consisting of groups from the UKE, the Heinrich Pette Institute and the BNITM and funded by the BWFG we now want to identify common immune mechanisms that might underlie the sex-dependent outcome in a variety of diseases.
Funding:
Landesforschungsförderung Hamburg; "Geschlechtsdimorphismus im Immunsystem: Bedeutung für Erkrankung und Immunität" (LFF-FV45)