Unraveling the complex interaction between Plasmodium falciparum infected erythrocytes and its host
Johannes Allweier, Jakob Cronshagen, David Danicic-Rauchberger, Florian Kieferle, Nahla Galal Metwally, Susann Ofori
Introduction
Plasmodium falciparum is responsible for the majority of malaria-related morbidity and mortality in humans. Of great importance to malaria pathology is the ability of P. falciparum-infected erythrocytes (PfIEs) to adhere to vascular endothelia. To date, little is known about the kinetics and dynamics of the cytoadhesion process, which depends mainly on a highly diverse protein family, P. falciparum erythrocyte membrane protein 1 (PfEMP1).
PfEMP1, which is presented on the erythrocyte surface, allows PfIEs to bind to various endothelial cell receptors (ECRs), thus disappear from the peripheral circulation and by removal by the spleen. The PfEMP1 family is encoded by approximately 45-90 var genes per parasite genome. Expression of var genes is mutually exclusive in ring-stage parasites, such that only a single PfEMP1 variant ever present on the surface of trophozoite- or schizonts-stage PfIEs at any given time. PfEMP1s are concentrated in nanoscale, electron-dense protrusions of the plasma membrane of PfIEs called knobs. Different PfEMP1s have different binding properties to ECRs and are associated with different clinical outcomes. Several studies have shown that severe malaria is associated with the expression of specific PfEMP1 groups, which include PfEMP1s that contain a binding domain for the endothelial protein C receptor (EPCR). In contrast, infections dominated by CD36-binding parasites show mild disease courses. Interestingly, between 75%-85% of PfEMP1s in a parasite isolate possess a binding domain for CD36.
Results
Using a laminar flow system, we showed that PfIEs roll over CD36, and the rolling behavior of disc-shaped PfIEs at the trophozoite stage (flipping) differs from the rolling behavior of round-shaped PfIEs at the schizont stage (continuous rolling). However, the binding mode of PfIEs also seems to be strongly dependent on the respective ECR. For ICAM-1, CD9, P-selectin, as well as chondroitin sulfate A (CSA), stationary binding, instead of rolling, was observed. Another important observation was made under fever conditions. Only knob-positive PfIEs were able to bind to different ECRs (CSA, CD36) at 40°C, preserving the ECR-specific binding mode (rolling or stationary). In summary, there is strong evidence that the presence of knobs on the surface of PfIEs is an essential requirement for the circulating parasite to adhere to the endothelium even under febrile conditions. Thus, there is an evolutionary pressure for the formation of knobs (Metwally et al., 2017; Lubiana et al.,2020; Dörpinghaus et al., 2020; Bachmann, Metwally et al., 2022).
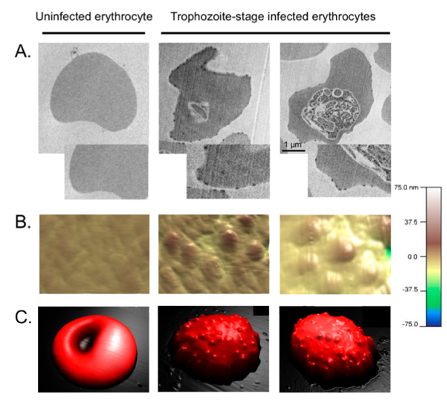
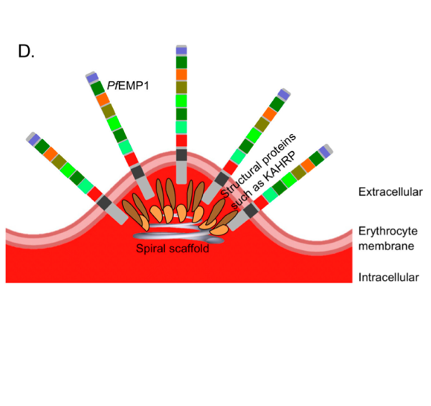
The knobs of PflEs. (A) Transmission electron micrographs. (B,C) Atomic force microscopic, three-dimensional images. (D) Simplified schematic illustration of the of knobs. From: Bachmann, Metwally et al., 2022.
Project A
Based on these results, we developed an alternative model for the process of cytoadhesion. In this model, removal of PfIEs from the bloodstream occurs via CD36, over which PfIEs subsequently move in a rolling fashion, leading to little or no activation of endothelial cells and favoring the development of mild malaria. As infection progresses, activation of the immune system occurs, leading to the presentation of additional ECRs. To these, static binding of PfIEs via PfEMP1s with other binding domains takes place, resulting to further stimulation of the immune system and favoring the development of severe malaria.
To test this model, we have P. falciparum transfectants that each present a specific PfEMP1 on the surface of PfIEs. Using these transfectants, it is possible to determine the binding profiles of the different PfEMP1s for different ECRs and to identify new binding partners. A laminar flow system will be used to visualise the binding behaviour (static/rolling) of the respective interaction partners. Furthermore, the binding of P. falciparum transfectants to primary endothelial cells from different organs will be analysed and the influence of cytoadhesion on endothelial cell gene expression will be determined by RNAseq. These studies will allow us to understand the effects of cytoadhesion of PfIEs to different endothelial receptors in different regions of the host.
Grey electron microscope movie taken in the S4-Lab showing cells and parasites ©BNITM
Project B
Little is known about the nature of the binding between PfEMP1s and ECRs. To analyse the binding properties, we have transgenic cells and plasmodia expressing defined ECRs or PfEMP1s. Atomic force microscopy (AFM) allows us to distinguish between single and multiple binding and between slip and catch binding mechanisms. Analysis using a surface acoustic wave biosensor and isothermal titration calorimetry allows characterisation of binding (association and dissociation). Direct visualisation of the interaction can be achieved using a combination of AFM/electron and immunofluorescence microscopy.
Funding:
Graduate School‚ Infection Biology of Tropical Pathogens‘, Joachim Herz Foundation. Joint PhD project (J. Cronshagen) with research group Malaria Cell Biology, BNITM (Head Tobias Spielmann).
DFG Priority program 2332: Physics of Parasitism. Joint PhD project (J. Allweier) with Thomas Gutsmann, Head Biophysics, Research Center Borstel, Germany.