Forschungsprojekte
Malaria stellt trotz erheblicher Anstrengungen zur Bekämpfung nach wie vor eine große Bedrohung für die öffentliche Gesundheit in tropischen Ländern dar. Die schwersten klinischen Verläufe sind auf Infektionen mit dem Einzeller Plasmodium falciparum zurückzuführen. Die hohe Virulenz von P. falciparum wird u. a. mit der Fähigkeit infizierter roter Blutkörperchen assoziiert an zahlreiche Oberflächenmoleküle wie CD36, EPCR und ICAM-1 des Blutgefäßendothels zu haften. Diese sogenannte Sequestrierung ermöglicht es dem Parasiten seiner Eliminierung in der Milz zu umgehen. Die vom Parasiten zur Sequestrierung produzierten Membranproteine sind jedoch dem Immunsystem des Wirts ausgesetzt und müssen bei einer langanhaltenden, chronischen Infektion durch einen als Antigenvariation beschriebenen Mechanismus ständig ausgetauscht werden. Aus diesem Grund besitzt P. falciparum Genfamilien, wobei jeweils mehrere Genkopien für unterschiedliche, polymorphe Membranproteine kodieren. Die bekannteste ist die hochmolekulare PfEMP1-Familie (P. falciparum erythrocyte membrane protein 1), die von 60 var-Genen pro Parasitengenom kodiert wird. Jedes var-Gen kodiert eine ähnliche, aber nicht identische PfEMP1-Version mit charakteristischen Rezeptorbindungseigenschaften. Durch das Umschalten der Genexpression zwischen verschiedenen var-Varianten kann der Parasit im Verlauf der Infektion sein immunologisches Erscheinungsbild und auch seine Zytoadhäsionseigenschaften verändern. Die Blockierung des Austauschs verschiedener PfEMP1-Varianten, die auf der Oberfläche infizierter Erythrozyten exponiert sind, würde demnach dem menschlichen Immunsystem einen großen Vorteil bei der Bekämpfung der Malariainfektion verschaffen. Darüber hinaus könnten krankheitsverursachende PfEMP1-Varianten oder andere Virulenzfaktoren als Interventionsziel zur Bekämpfung der Malaria genutzt werden.
Expression von Virulenzgenen in experimentellen humanen Malariainfektionen
Wie der Parasit seine var-Genexpression in vivo koordiniert ist von großem Interesse, und wir nutzen derzeit kontrollierte humane Malariainfektionen (CHMI), um dieser Frage nachzugehen. Bei CHMI-Studien werden entweder Malaria-naive oder semi-immune Freiwillige mit Sporozoiten, dem infektiösen Mückenstadium des Parasiten, infiziert, die in unserem Fall von Sanaria Inc. bereitgestellt werden. Parasiten-positives Blut der Freiwilligen wird sofort für die Erstellung von Transkriptionsprofilen des gesamten var-Genrepertoires mittels quantitativer RT-PCR (qPCR) oder RNA-Sequenzierung verwendet. Zum Vergleich werden auch in vitro kultivierte "Prä-Moskito-Parasiten", Gametozyten und Sporozoiten analysiert. Durch einen Vergleich der Genexpression zwischen (i) verschiedenen Parasitenstadien, (ii) in vitro kultivierten und ex vivo-Parasiten, (iii) Parasiten von Malaria-naiven und semi-immunen Probanden, (iv) Proben, die im Verlauf der Infektionen entnommen wurden, und (v) genetisch unterschiedlichen und geografisch entfernten Parasitenstämmen können wir Strategien des Parasiten zur Etablierung und Aufrechterhaltung der Infektion bei Individuen mit unterschiedlichen Immunitätsgraden aufdecken.
Publikationen:
Wichers-Misterek et al. 2023 PLoS Pathogens; DOI: 10.1371/journal.ppat.1011468.
Bachmann et al. 2019 PLoS Pathogens; DOI: 10.1371/journal.ppat.1007906.
Bachmann et al. 2016 PLoS Pathogens; DOI: 10.1371/journal.ppat.1005538.
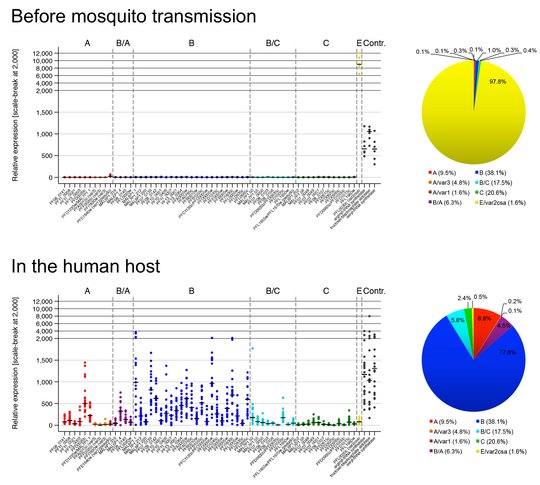
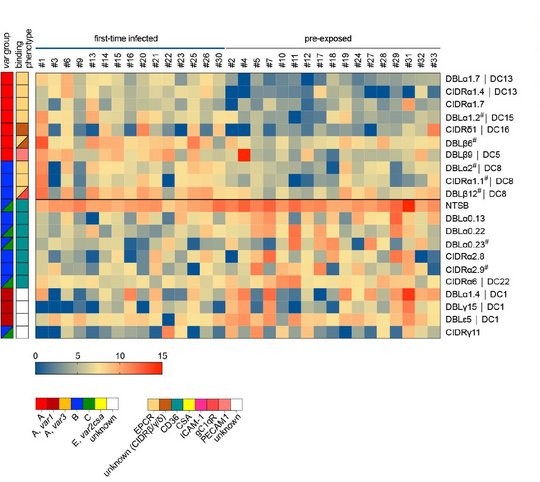
©Anna Bachmann
Expression von Virulenzgenen in Malariapatienten
In Malaria-Endemiegebieten leiden vor allem Kinder unter fünf Jahren und schwangere Frauen an schwerer Malaria, wobei auch Erwachsene in Gebieten mit geringerer Endemie und nicht-immune Reisende anfällig für schwere Erkrankungen sind. Obwohl sich schwere Malaria bei Kindern in drei sich überschneidenden Syndromen manifestiert (zerebrale Malaria, Azidose und schwere Anämie), variiert die Krankheitssymptomatik auch nach Alter, wobei Erwachsene mehr Multiorgan-Komplikationen und eine höhere Sterblichkeitsrate aufweisen. Die Faktoren, die für die verschiedenen Krankheitssyndrome bei Kindern oder die altersbedingten Unterschiede bei Erwachsenen verantwortlich sind, sind noch weitgehend unbekannt. Um die biologischen Prozesse besser zu verstehen, die an den Interaktionen zwischen Wirt und Erreger beteiligt sind, analysieren wir zum einen (i) Proben einer ghanaischen Kohorte pädiatrischer Malariapatienten mit gut definierten klinischen Phänotypen der schweren Malaria im Vegleich zu Proben von asymptomatischen Kontrollpersonen, zum anderen (ii) Proben von erwachsenen Reisenden mit variierender vorheriger Malariaexposition und unterschiedlichen klinischen Manifestationen. Wir verwenden eine von uns etablierte RNA-Sequenzierungs- und Analyse-Pipeline, um Assoziationen zwischen der Genexpression der Parasiten (einschließlich var-Gene) und den verschiedenen Malariasyndromen, dem Schweregrad der Erkrankung und dem Immunstatus aufzudecken.
Publikationen:
Andradi-Brown et al. 2023 eLife (reviewed preprint); DOI: 10.7554/elife.87726.1.
Wichers et al. 2021 eLife; DOI: 10.7554/elife.69040.
Genexpression, subzelluläre Lokalisierung und Funktion kleiner Oberflächenantigene
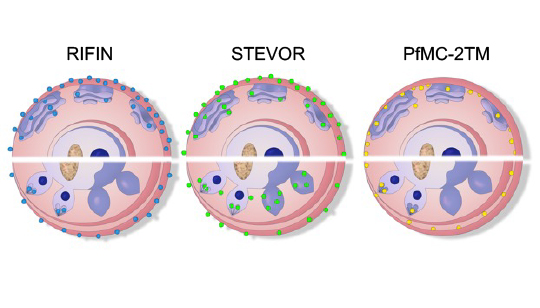
Neben dem bekanntesten Oberflächenantigen PfEMP1 gibt es die weniger gut charakterisierten sogenannten kleinen Oberflächenantigene RIFIN, STEVOR und PfMC-2TM auf P. falciparum-infizierten roten Blutzellen. Interessanterweise wurden sie jedoch auch an anderen Stellen innerhalb infizierter roter Blutzellen lokalisiert, so dass sie vermutlich multiple Funktionen ausüben. So wurden sie funktionell auch schon der Immunregulation, dem Rosetting und auch der Merozoiteninvasion in Verbindung gebracht. Ein größeres Wissen über diese kleinen Oberflächenproteine ist jedoch notwendig und könnte neue Angriffspunkte für Interventionen aufzudecken. Um einen besseren Einblick in ihre Funktion zu erhalten, haben wir Analysemethoden für Parasiten von Malariapatienten entwickelt, deren Genexpression und Proteinlokalisierung nicht durch eine längere In-vitro-Kultivierung beeinflusst wird. Wir konnten bereits zeigen, dass die Genexpression von RIFIN- und STEVOR-kodierenden Genen in vivo deutlich erhöht ist und dass Proteine beider Familien an die Oberfläche infizierter roter Blutkörperchen transportiert werden. Derzeit charakterisieren wir die Genexpression, die Lokalisierung, die Interaktionspartner und die Funktion der kleinen Oberflächenantigene in unterschiedlichen Lebenszyklusstadien des Parasiten.
Publikationen:
Wichers et al. 2019 mBio; DOI: 10.1128/mbio.01500-19.
Bachmann et al. 2015 Malaria Journal; DOI: 10.1186/s12936-015-0784-2.