Research Projects - Virology
Epidemiology and immunology of human bornavirus encephalitis
Human bornavirus encephalitis is a severe disease caused by two related zoonotic members of the Bornaviridae family, the emerging Borna disease virus 1 (BoDV-1; species Mammalian 1 orthobornavirus), and the rarely detected variegated squirrel bornavirus 1 (VSBV-1; species Mammalian 2 orthobornavirus). While human BoDV-1 encephalitis cases are increasingly detected and have been described in regions endemic for animal Borna disease (BD) in Germany, human VSBV-1 infection is so far limited to five confirmed cases in private holdings and zoological gardens, and acquired through contact to exotic squirrels. The portal of entry and the respective transmission routes to humans are unknown and subject to ongoing research studies.
The group is part of ZooBoCo (Zoonotic Bornavirus Consortium), a BMBF-funded research network, and conducts molecular, epidemiological and immunological studies on human bornavirus encephalitis. For a list of the group's publications about bornavirus encephalitis, click here. For more background information, and details for diagnostic procedures, follow this link (in German).
Borna disease virus 1 (BoDV-1)
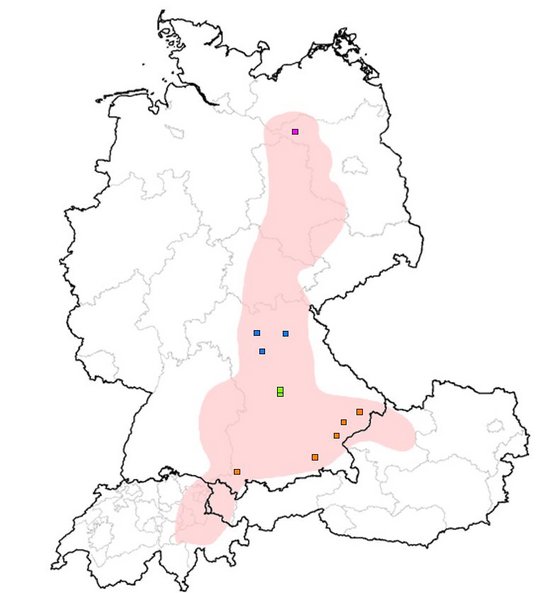
For a long time, BoDV-1 has been noted to cause animal BD, a non-purulent meningomyelo-encephalitis of mainly horses and sheep in endemic regions of Germany, Liechtenstein, Switzerland and Austria. BoDV-1 is harbored by at least the insectivorous bicolored white-toothed shrew (Crocidura leucodon) as a natural reservoir. The shrews are asymptomatic and shed the virus by feces and urine. In 2018, the severe pathogenic potential of BoDV-1 for humans became apparent in a cluster of transplant-related BoDV-1 encephalitis cases in Germany with two fatalities; simultaneously, one sporadic unrelated fatal case was detected. Since then, more than 50 sporadic cases, some acute and some retrospectively diagnosed, have been published and/or were notified to public health authorities. It is suspected that most patients become infected close to their rural places of residence.
Variegated squirrel bornavirus 1 (VSBV-1)
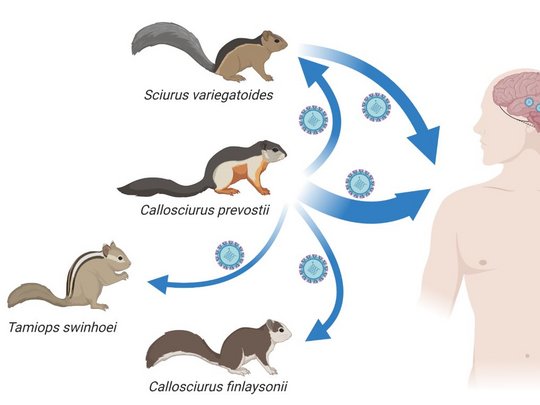
VSBV-1 was detected in 2015 as the cause of fatal encephalitis in three private breeders of exotic variegated squirrels (Sciurus variegatoides) in the East of Germany. In 2018 and 2021, VSBV-1 was found to be responsible for the fatal encephalitis of two zoo animal caretakers in the North of Germany who had cared for another exotic squirrel species, Prevost’s squirrels (Callosciurus prevostii). Investigations in private holdings and zoological gardens in Germany and elsewhere in Europe revealed VSBV-1 detection rates of 1.5% - 8.5% in captive exotic squirrels. The animals are asymptomatic and show high viral loads in the brain, kidneys, urinary bladder, skin, and oral cavity, thus qualifying them as potential natural reservoirs. The origin of the virus and its route of introduction into the captive squirrel populations in Europe remained to be elucidated.
Epidemiological studies of BoDV-1 encephalitis
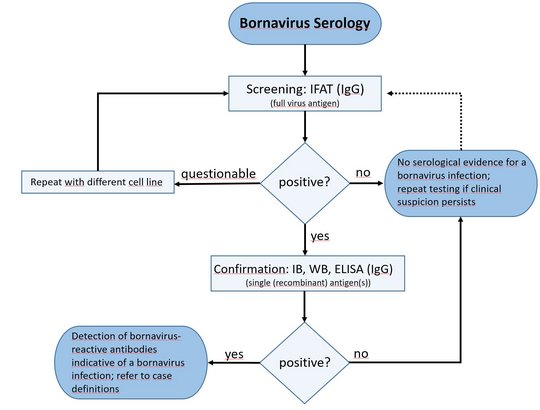
Several retrospective and prospective studies for active case finding, as well as cross-sectional studies for seroprevalence investigations were conducted, employing validated testing schemes and adhering to specific case definitions.
Human BoDV-1 cases appear rare, even in endemic areas, but show a high case-fatality ratio. Among several hundred tested human patients, BoDV-1 infections were only detected among those with encephalitis and not in patients suffering from other, partly unspecified, diseases.
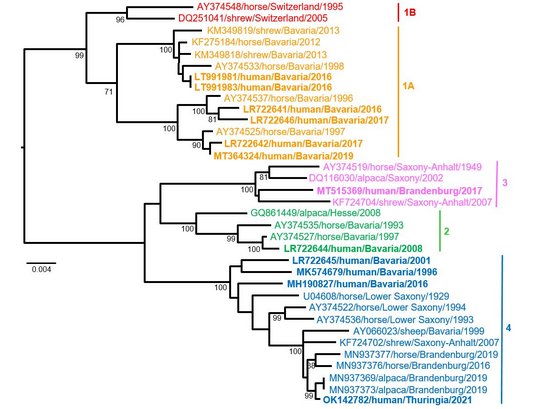
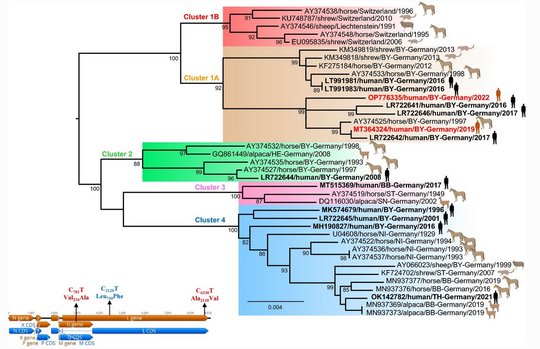
Virus sequences from patients matched closely those from shrew reservoirs, revealing several viral clusters within the BD endemic area and reflecting spill-over transmission close to the patient’s often rural place of residence.
As a consequence, BoDV-1 infection has to be routinely considered as differential diagnosis in human encephalitis cases in all regions endemic for animal BD and areas known to harbor BoDV-1 positive shrews. An assumed incubation period of a few months may mean that acute BoDV-1 encephalitis could present outside of BD endemic areas as well. The direct detection of bornaviruses pathogenic for humans became notifyable in 2020 in Germany.
Human cases were so far found in Bavaria, Brandenburg, Thuringia and Saxony-Anhalt.
For a list of the group's publications about BoDV-1, click here.
Risk factors and possible portal of entry for BoDV-1
The first comprehensive epidemiological risk factor analysis, a large case series combined with a matched case-control study, revealed that residence close to nature in a stand-alone location or on the fringe of the settlement in virus-endemic areas is a risk factor with an adjusted Odds Ratio of 11.
Except for the household locations, the profession, animal contacts, specififc outdoor activities, travel, and nutrition did not play a role for infection. None of the interviewed households recalled a transmission event such as direct shrew contact. However, peridomestic shrew presence was confirmed in 60% of cases, thus supporting environmental transmission. So far, a seasonality is not obvious in human cases.
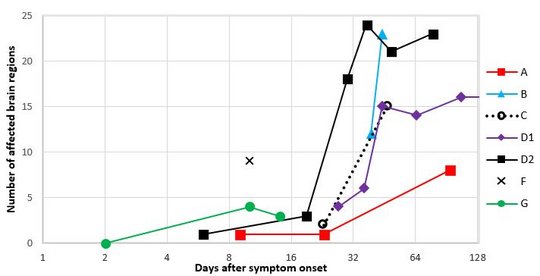
Currently, a local clustering of cases is seen in Germany. The first and most prominent cluster comprised two children in a small village who were infected three years apart. Patients lived 750m away from each other and on the fringe of the settlement, a recently shown risk factor.
The two human-derived BoDV-1 sequences were not identical, indicating viral diversity in natural reservoirs. Specific transmission events or a common source of infection were not found. Continuing prospective studies and surveillance will show whether case clusters reflect hot spots or endemicity.
While the first case was diagnosed late in the course of disease, a very early diagnosis and treatment attempt facilitated by heightened awareness was achieved in the second case. Following initial impressive clinical improvement, the course was also fatal, although clearly prolonged.
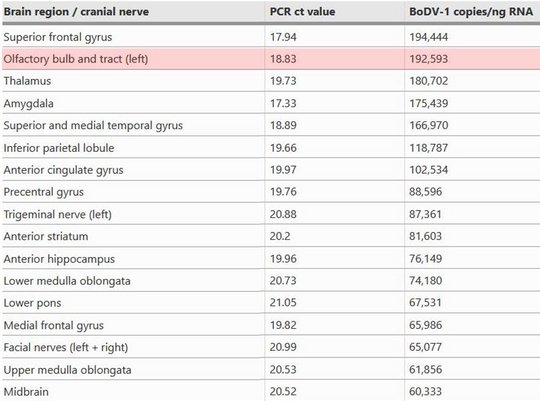
Viral RNA was detected by qPCR in tear fluid and saliva, constituting a possible transmission risk for health care professionals. Highest viral loads were found post mortem in the olfactory nerve and the limbic system, possibly reflecting the portal of entry for BoDV-1. Whole exome sequencing in both patients yielded no hint for underlying immunodeficiency.
Targeted prevention, future post-exposure-prophylaxis, and timely diagnosis remain challenging.
For a list of the group's publications about BoDV-1, click here.
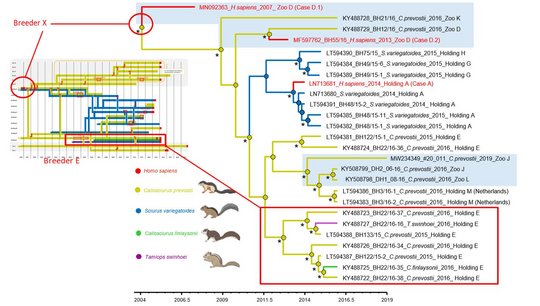
Host switching events were detected as dominant evolutionary mechanisms driving the virus-host associations. Virus spread by animal trade followed by subsequent local micro-evolution in zoos and holdings is responsible for diversifying strains. While zoos had kept predominantly Prevost’s squirrels, private breeders partly had kept variegated squirrels and Prevost’s squirrels in close proximity, resulting in an enhanced probability of cross-species transmission.
Understanding the epidemiology of VSBV-1 spread is crucial to develop and implement surveillance strategies for control. The studies underscore the risk of VSBV-1 transmission in private and occupational settings and the need for monitoring of all exotic squirrel holdings. Examinations of wild exotic squirrel populations and investigations of human encephalitis cases of unknown aetiology for VSBV-1 infection in the tropics may shed more light on the epidemiology and the geographic origin of VSBV-1.
For a list of the group's publications about VSBV-1, click here.
Epidemiological studies of VSBV-1 introduction and spread
Epidemiological and serological investigations were performed in various German zoos and among squirrel breeders, leading to the discovery of further confirmed, probable, and possible human cases. A complex, intermingling trading network involving several squirrel species, private animal breeders/keepers and zoological gardens was investigated.
The studies provide insight into the spread of this novel viral pathogen among different exotic squirrel species in captivity and spill-over infections to humans.
The striking genetic homogeneity of all currently known VSBV-1 genomes suggests a single introduction of the virus into the known captive exotic squirrel populations in Europe, which is supported by consolidation of phylogenetic analysis and trade network investigations. Phylogenetic data indicate that the initially infected animals in the here identified network were Prevost´s squirrels.
Pathophysiology and immunological studies of bornavirus encephalitis
Bornaviruses are not cytolytic, and the severe tissue destruction seen during encephalitis by either virus is immunopathgenetically induced.
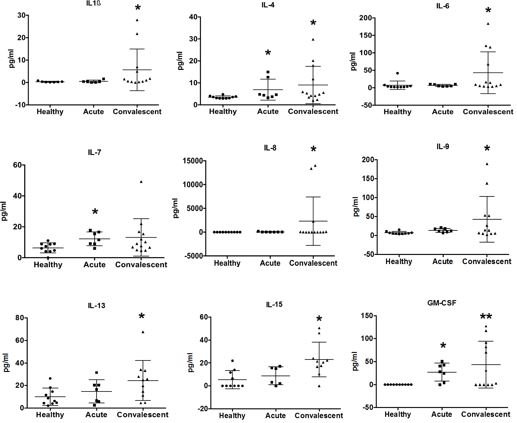
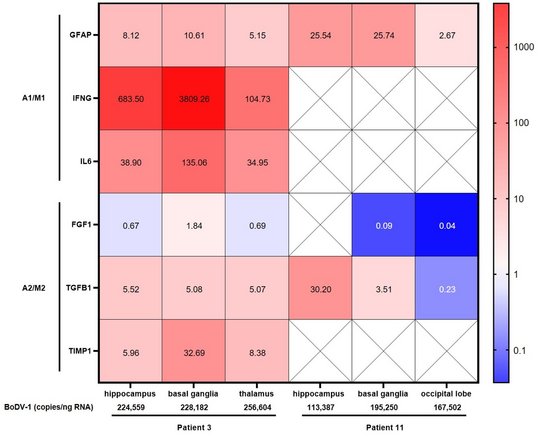
For BoDV-1, a marked and increasing immune activation during encephalitis is shown in serum and CSF. A pro-inflammatory state as part of the immuno-mediated pathology, shown by elevated respective cytokines, chemokines and other biomarkers, dominates during the course of human BoDV-1 encephalitis. IFNγ production was demonstrated in endothelial cells, astrocytes and microglia, IL-6 in activated microglia, and TGF-β1 in endothelial cells, activated astrocytes and microglia. Pathologically low growth factor levels were seen. This dysbalanced, pro-inflammatory state likely contributes to the fatal outcome of human BoDV-1 encephalitis, and might be a key target by immunusuppressants for treatment attempts in addition to antivirals.
Such proinflammatory activation states can be experimentally induced in brain organoid models. BoDV-1 infects neurons, astrocytes and oligodendrocytes. These cell populations are present in the complex three-dimensional models as well, and insights in viral spread and pathophysiology may be gained from organoid infections.
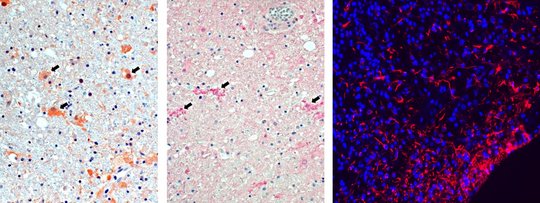
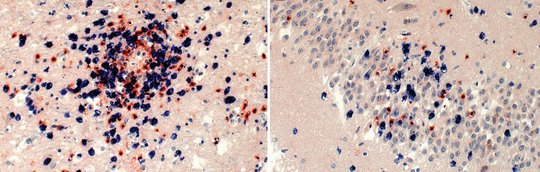
For VSBV-1, and similar to BoDV-1 analyses performed by others, inflammatory infiltrates in areas positive for viral RNA and antigen consist of CD4+ and CD8+ T cells, with perivascular B-cell accumulation. Strong microglial response and bizarre astroglial expansion were present. Hallmarks of apoptosis were seen, such as cleavage of caspase 3 in cells adjacent to CD8+ cells and widespread p53 expression.
Highest viral loads were found in the basal ganglia, thalamus, and the limbic system. These are the regions that are first and preferably shown to be infected in imaging studies (see below).
A list of the group's publications about bornavirus immunopathology can be found here.
Imaging of VSBV-1 encephalitis
Magnetic resonance imaging (MRI) scans demonstrated characteristic T2 hyperintense lesions in the limbic system and the basal ganglia, followed by the brainstem, in VSBV-1 encephalitis patients. No involvement of the cerebellar cortex was seen. Deep white matter affection occurred in a later stage of the disease. Sinusitis in three patients on the first MRI and an early involvement of the limbic system suggest an olfactory route of VSBV-1 entry. The viral spread could then occur to adjacent anatomical brain regions or along specific neural tracts to more distant brain regions.
The overall pattern closely resembles that described for BoDV-1 encephalitis. The exact bornavirus species can thus not be deduced from imaging results alone, and molecular testing and serology should be performed to confirm the causative bornavirus. As VSBV-1 is likely of tropical origin, and MRI investigations are increasingly available globally, imaging techniques might be helpful to facilitate an early presumptive diagnosis of VSBV-1 encephalitis when molecular and/or serological testing is not available.
Research Projects - Virology
Clinical and immunological analyses of arbovirus infections
Several individual cases, case series, and cohorts with various arboviral infections were analyzed clinically, imunologically, and virologically. Passive surveillance techniques enabled us to detect and examine the first imported Zika virus infections in Europe, as well as other unusual arbovirus diseases, including those caused by O'nyong-nyong, Ross River and Mayaro viruses.
Alphaviruses cause long-lasting arthralgia after the acute phase of infection. Our analyses of several clinical cohorts demonstrate prolonged elevated proinflammatory cytokine levels in the recovery phase of arthritogenic alphaviral disease. Cytokine level testing in alphavirus infections with prolonged arthralgia may aid monitoring patients' conditions, especially when clinical signs of arthritis (joint swelling and redness) are no longer present, and standard serum inflammatory parameters are within reference ranges.
For a list of the group's contributions to arboviral research click here.