Outbreak Preparedness and Response Projects
Our group (OPR) is dedicated to strengthening both stationary and mobile laboratory capacities for viral hemorrhagic fevers and other outbreak-prone viral diseases in Sub-Saharan Africa. Our broad laboratory expertise, encompassing diagnostics, serology, field sequencing and quality assurance management, aims to offer a global solution to prepare for and rapidly respond to the emergence of pathogens in endemic countries. We are also actively involved in providing laboratory support to large epidemiological studies and clinical trials.
Our work includes operational research, clinical trials as well as capacity building. Key projects are the European Mobile Laboratory (EMLab) and the cooperation with the Coalition for Epidemic Preparedness Innovations (CEPI).
Capacity Building
To reduce the burden of viral hemorrhagic fevers in Sub-Saharan Africa, early detection and integrated laboratory response are key! To support this, we strengthen laboratory capacities where needed through long-term cooperation’s or punctual missions. Diagnostics, serology, quality assurance management and field sequencing are part of our portfolio offering a global solution to prepare for and rapidly respond to such threats.
Long-term cooperations
Our capacity building programs aim at strengthening the laboratory workforce of cooperation partners in West Africa, to deliver high quality, in-country surveillance data for viral pathogens up to risk group 4. We aim to offer a global solution to prepare for and rapidly respond to the emergence of pathogens in endemic countries. Furthermore, we strive to use this surveillance information to inform public health responses and support the assessment of countermeasures for the benefit of the populations. The 2014-2016 outbreak of Ebola virus disease (EVD) in West Africa, the COVID-19 pandemic and the multi-country emergence of Mpox (formerly Monkeypox) are examples of the extreme threat emerging or re-emerging infectious diseases pose to the global community. Climate change and increased contact with wildlife and other potential vectors through environmental encroachment create an even higher risk for infectious diseases outbreaks. Significant efforts are thus needed to improve laboratory preparedness and response to public health emergencies.
For many years we have been closely working with our cooperation partners from Nigeria, Guinea and Benin to reinforce national diagnostic capacity for Lassa, Ebola, Marburg, and Yellow Fever viruses among others. Furthermore, in line with the “Global Genomic Surveillance Strategy” of the World Health Organization (WHO), our programs focus on implementing field sequencing laboratories. Building on the successful establishment of SARS-CoV-2-sequencing capacities in Guinea (2021) and Nigeria (2022), we are now expanding such genomic surveillance capacities to include identification of a broad range of other life-threatening viral pathogens. Our capacity building programs have yielded key publications in the field of viral hemorrhagic fever surveillance.
Our team offers tailored training programs and deploys several times per year to partner countries. We also support the academic careers of cooperation partners. These activities are mostly supported by the Global Health Protection Program (GHPP) of the German Ministry of Health (AfroLabNet 2.0 and CELESTA).
Contacts:
- Benin: Dr Meike Pahlmann
- Guinea (all sites) and Nigeria (sequencing): Dr Giuditta Annibaldis & Dr Sophie Duraffour
- Nigeria: Dr Meike Pahlmann & Dr Lisa Oestereich
- Sierra Leone: Dr Meike Pahlmann


Expertise at short notice, flexibly, professionally, and globally
As a WHO Collaborating Center, a Global Outbreak Alert and Response Network (GOARN) and German Epidemic Preparedness Team (SEEG) partner institutions, experts from our team can deploy in response to infectious diseases outbreaks or preparedness activities following a request for assistance from a country. We offer a broad range of laboratory-related expertise and related training programs in the field of diagnostics, serology and sequencing of viral hemorrhagic fever viruses and other outbreak-prone viral diseases for the benefit of the requesting country.
Follow the links for more information about GOARN and SEEG.
Contacts:
- GOARN focal points: Dr Sophie Duraffour & Prof Dr Stephan Günther
- GOARN Deputies: Dr Giuditta Annibaldis & Dr Emily Nelson
- SEEG: Dr Meike Pahlmann & Dr Kathrin Schuldt
Operational research
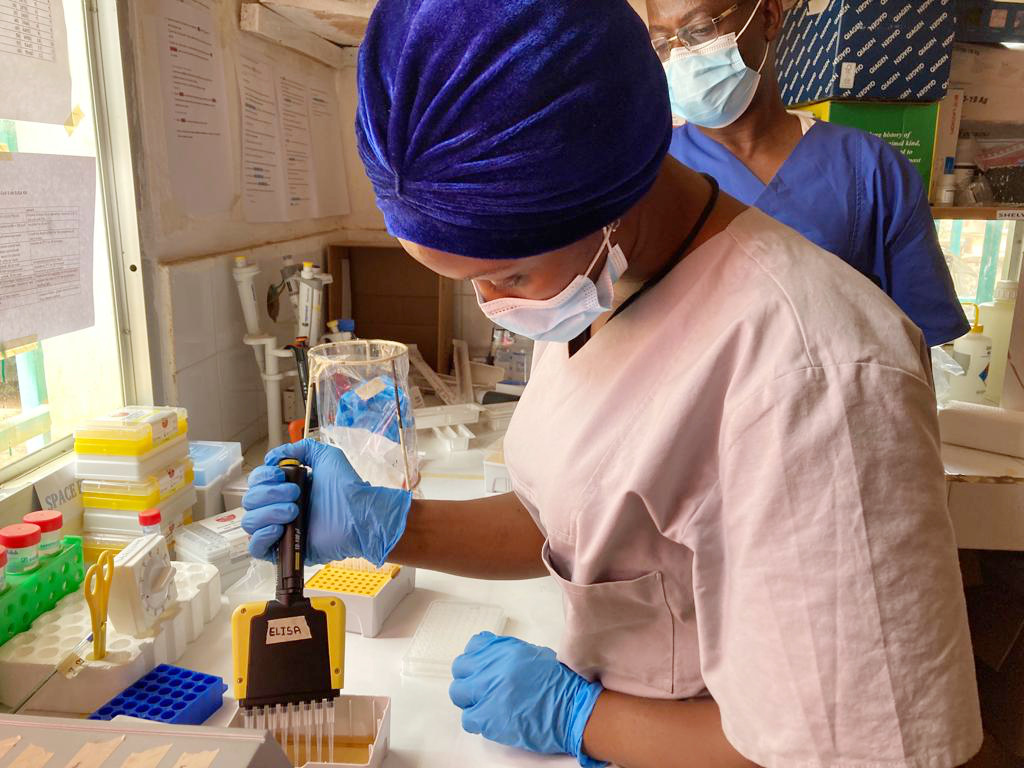
Understanding the circulation, emergence and persistence of viral hemorrhagic fevers is key to ultimately support the development of countermeasures and save lives. Our group deciphers these crucial research gaps by looking into both disease occurrence in West Africa and virus genome evolutionary dynamics. Find out more here.
We are interested in monitoring and understanding viral hemorrhagic fever emergence, patterns of transmission (i.e., molecular epidemiology), virus persistence and virus genome evolutionary dynamics. Our operational research particularly focuses on Lassa fever, Ebola virus disease, Marburg virus disease and COVID-19. In addition to outbreak response activities, we also have several community and observational clinical studies on-going in Nigeria and Guinea which support the research being done both in partner countries and at the BNITM. Our interests range from understanding how diseases circulate in populations (mainly through sero-survey studies) to understanding virus genome evolution both inter and intra-hosts. Next generation sequencing represents a large part of our work. To reach this goal, we combine the expertise of several BNITM groups and external collaborators in the field of immunology, epidemiology, ecology, clinical studies, diagnostics development, and computational and evolutionary virology. Our previous work has already addressed major gaps in this field and has led to several high-ranking publications. We ultimately wish to contribute to outbreak control efforts in endemic regions by guiding public health policies and to the development of modern molecular diagnostics as well as prophylactic and therapeutic strategies.
Contact: Dr Sophie Duraffour
Clinical Trials
The development of innovative treatment options against Lassa fever is a core aspect of our activities. We closely collaborate with several partners to establish clinical trial capacities in West Africa. Discover here our successful achievements and upcoming missions.
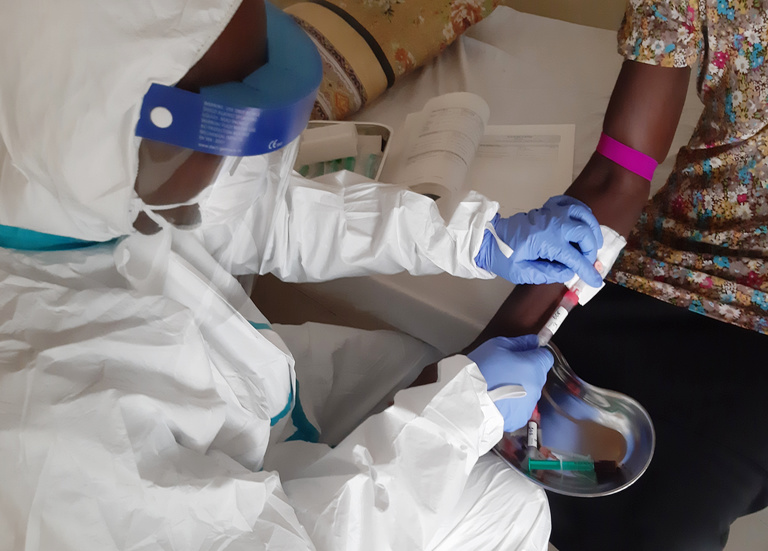
Building on our long-term laboratory capacity building programs with the Irrua Specialist Teaching Hospital (ISTH) in Nigeria, we expanded our collaboration together with the Clinical Research Department of the BNITM to build up local expertise in conducting clinical trials with the ultimate goal of improving the treatment of Lassa fever. As of today, 20% of people infected with Lassa virus die from the disease and there are still no vaccines or approved drugs with regulatory approval for Lassa fever treatment. As part of these programs, prophylactic or therapeutic options are being investigated, new partnerships established and local capacities reinforced to run clinical trials.
In 2020, we successfully completed a prospective observational study to evaluate the pharmacokinetic of ribavirin in Lassa fever patients (study acronym: PAIRR). This provided the ground to initiate a randomized phase II clinical trial to evaluate a drug candidate (favipiravir) alone and in combination with ribavirin (clinical trial acronym: SAFARI). To strengthen Nigeria's capacity, an additional clinical trial site was set up at the Federal Medical Center of Owo (Ondo State), and the collaborative network was further extended to the “Institut National de la Santé et de la Recherche Médicale” (INSERM), the Université Bordeaux, and the Alliance for International Medical Action (ALIMA). In December 2022, the SAFARI study was successfully completed and a new phase II clinical trial is planned for 2023.
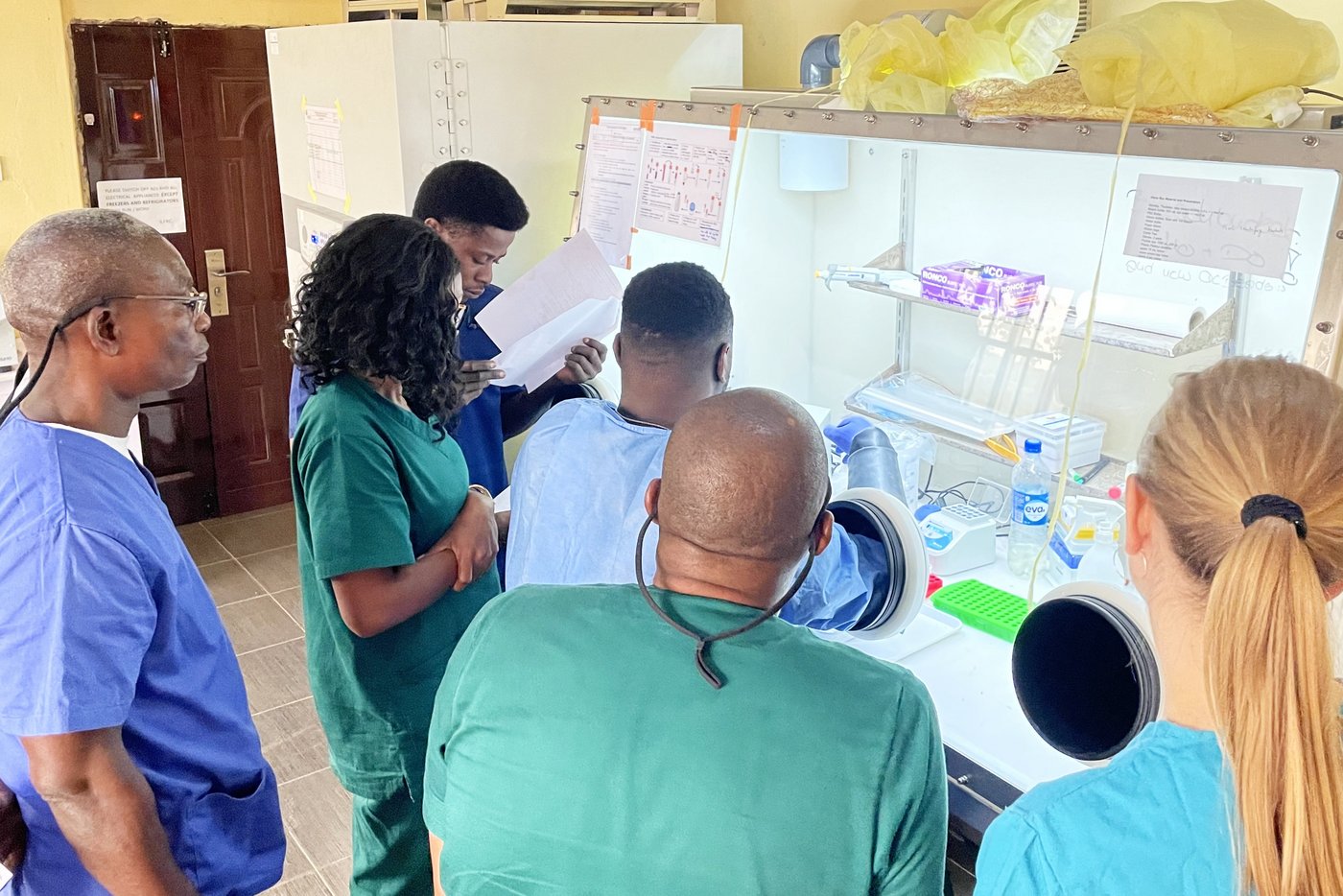
Another 2022 milestone was achieved with the creation and the launch of the INTEGRATE consortium to ease the identification of therapies for Lassa fever. The international consortium consists of leading scientists, physicians, institutions, non-governmental organizations, funders, and policymakers from several institutions from the Global South and North with key expertise in the required field. Our group is part of the scientific board of this global alliance against Lassa fever. The consortium aims to establish a versatile, GCP-compliant clinical trial platform in West Africa for the development of medical countermeasures against Lassa fever. This will be accomplished through capacity development, technology transfer, and training. To prepare for this, a Site Preparedness Program (SPP, funded by PANTHER from the Federal Ministry of Research and Education in Germany) has been launched to potentially integrate new cooperation partners into the consortium. Through INTEGRATE, our group reinforces its position as one of the major actors in the field of Lassa fever clinical trial development.
For more information on our Lassa work in Nigeria, click here.
Contacts:
INTEGRATE: Dr Meike Pahlmann & Dr. Ndapewa Ithete
SAFARI: Dr Meike Pahlmann
EMLab
When diseases like Ebola or Marburg strike, fast and accurate detection is crucial! The European Mobile Laboratory (EMLab) swiftly deploys advanced field laboratories and highly trained experts to support outbreak control efforts where needed. Discover more here.
The European Mobile Laboratory (EMLab) responds worldwide to disease outbreaks through the deployment of state-of-the-art field laboratories used for the detection of pathogens up to risk group 4, such as Ebola virus. EMLab is headquartered at the BNITM and managed through the department of Virology and the Mobile Laboratory Service Unit of the institute. Operations have evolved over the years to include not only molecular detection methods such as PCR/RT-PCR but also basic clinical care and viral genomic surveillance capacities. Since its first deployment in 2014, EMLab has been a key asset in responding to many outbreaks including Ebola virus disease, Yellow Fever, Marburg virus disease, Lassa Fever and COVID-19. It has deployed to a number of countries across Africa and Europe during public health emergencies including Guinea, Liberia, Sierra Leone, Nigeria, Uganda, the Democratic Republic of the Congo, Germany, and Greece.
EMLab is a partner of the WHO Global Outbreak Alert and Response Network (GOARN), including the WHO GOARN Rapid Response Mobile Laboratory (RRML) initiative, and is part of the European Civil Protection Pool (ECPP). The EMLab network is comprised of many partner institutions and experts from across the world. All staff deployed through EMLab receive specific mobile laboratory training and are part of our global laboratory expert network. EMLab actively participates in RRML and ECPP mock deployment exercises and may contribute to other consortia and projects such as the federally funded DigiPREW project, which aims to implement the Surveillance, Outbreak Response Management and Analysis System (SORMAS) for mobile laboratories.
Follow the links for more information on the EU Medical Corps.
Contact: Dr Emily Nelson




CEPI funded projects

The Coalition for Epidemic Preparedness Innovations (CEPI) works to accelerate the development of vaccines against epidemic and pandemic threats. Since 2019, our lab team lends its expertise within this global coalition efforts to fight Lassa fever.
In 2019, our team managed the CEPI serum collection project in Nigeria (2019-2021), of which the main objective was to collect serum from Lassa fever survivors for the development of an Immunoglobulin G (IgG) antibody standard for Lassa diagnostics. Since 2020, the ENABLE program is funded by CEPI to gather detailed data about the incidence of Lassa virus infections in five West African countries (Benin, Guinea, Liberia, Nigeria, Sierra Leone) and to provide information for designing future vaccine trials and vaccination strategies. This is the largest Lassa Fever study to date. This program also helps to strengthen the capacity of trial sites and investigators to conduct vaccine trials and addresses several gaps identified in the World Health Organization (WHO) Lassa Fever Research and Development (R&D) Roadmap.
The Virology department of the BNITM is the laboratory coordinator of the ENABLE program and our BNITM CEPI team provides key laboratory expertise for its successful implementation. Together with other coordinating partners including P95, the Margan Clinical Research Organization (MMARCRO) and MSF Epicentre, the BNITM team supports nine implementing partner sites across these five countries with harmonized laboratory processes, ranging from sample collection to safe sample handling and further downstream analysis, trainings and quality assurance management tools. Another ongoing CEPI project aims to evaluate the clinical performance of the RealStar® Lassa virus RT-PCR 2.0 kit (altona Diagnostics, Hamburg, Germany) in Sierra Leone.
For more information click here.
Contacts:
CEPI ENABLE: Dr Ndapewa Ithete & Dr Nathalie Vielle & Dr Danny Noack
CEPI-serum and CEPI-clinical performance: Dr Ndapewa Ithete
The European Virus Archive
In an interconnected world where emerging and re-emerging viral outbreaks pose significant threats, collaboration and resource sharing are indispensable for effective preparedness and response. The European Virus Archive - Global (EVAg) is an ambitious, multidisciplinary consortium comprised of a wide range of academic institutions across the globe that are at the forefront of human, animal and plant research in virology. EVA's collaborative network not only enhances outbreak control but also promotes the development of innovative tools, diagnostics, and therapies. By fostering cooperation, EVA empowers scientists to combine their expertise, pool resources, and collectively tackle the challenges posed by emerging and re-emerging viral diseases. The project promotes equitable access to resources and the acceleration of scientific progress by creating a centralized platform that provides access to a vast collection of viruses, associated materials, and cutting-edge tools. This comprehensive project encompasses multiple dimensions, focusing on the fair and equitable access to scientific resources, adherence to the Nagoya Protocol, robust quality management practices, and state-of-the-art biobanking methodologies.
Notably, the EVAg project integrates 13 biosafety level 4 (BSL4) facilities with expertise in the handling, research, and diagnostics of high-risk pathogens such as Ebola virus, forming the largest BSL4 network worldwide. The BNITM is part of this BSL4 network and manages a large collection of virus stocks and other derived products that it provides to collaborators from across the world. As part of this comprehensive network of BSL4 laboratories and adherence to stringent quality, ethical, safety, and security standards, EVAg and BNITM ensure that researchers have reliable and high-quality resources at their disposal.




























