Role of human miRNA in the pathogenesis of Malaria
Principal Investigator: Nahla Galal Metwally 🇪🇬 (CV)
Funding:
LCI (Leibniz center of infection) , Jürgen Manchot Stiftung, DZIF (German center for infection research)
PhD scholarship applications are welcomed (DAAD, Jürgen Manchot, etc..), for this please, drop me an email (metwally@bnitm.de).
Establishing the causal role of any single mechanism in severe malaria in humans is difficult. The pathogenesis of severe malaria is hotly debated; some suggest that cytoadhesion is the overriding pathogenic mechanism, whilst others believe that inflammatory processes are more important. An accumulating body of evidence indicates that vascular endothelial dysfunction is also important and could be the interface between cytoadhesion and inflammation.
Several cellular signaling pathways, including immune signaling pathways, need to be fine-tuned during infection, and some of these are regulated by miRNAs. miRNAs are non-coding RNAs of ~20 nucleotides in length that occur in mammals, plants and viruses. The human genome is estimated to contain 2300 true human mature miRNAs, of which 1115 are currently annotated, according to miRBase version 22
Our proposed theory
Our hypothesis is that the dysfunction of ECs is a trigger for severe malaria complications. This disrupts the balance between vasoconstriction and vasodilatation and predisposes to cytoadhesion of iRBCs, endothelial proliferation and leakage of the BBB. By regulating gene expression within ECs, certain miRNA candidates may be involved in controlling these events. These miRNAs may sense the presence of IEs through cell-cell communication between iRBCs and ECs.
Our aim
In this project, we scan the human miRNA profiles in the different cells that are involved during the major events of severe P. falciparum infection and cerebral malaria. The experiments are designed to monitor the first steps and role of parasite or host factors in triggering complications.
Team
Hanifeh Torabi (PhD student)
Marlena Kemper (MD student)
Viktor Michaelis (Master Student)

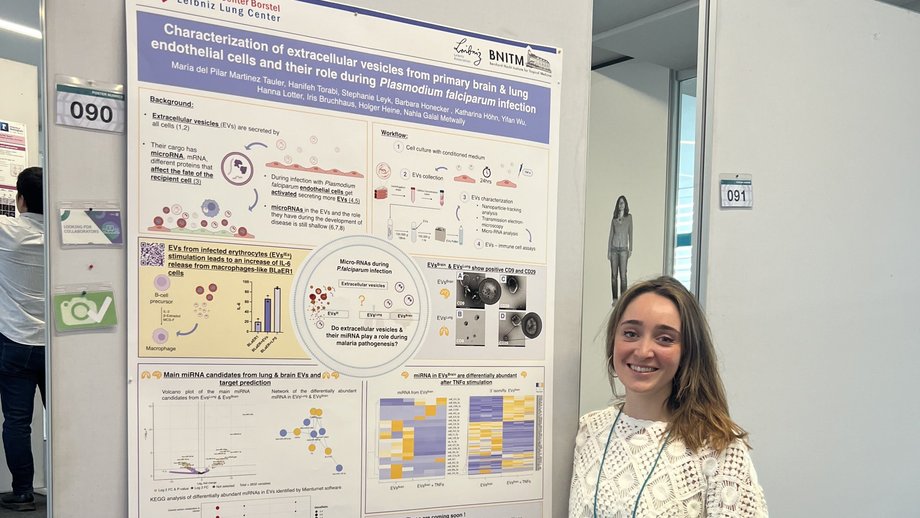
miRNA profiles of human brain and lung endothelial cells and their extracellular vesicles during Plasmodium falciparum infection
Pilar Tauler 🇪🇸, Nadja Götz 🇩🇪 , Hannifeh Torabi 🇮🇷 , Nahla Galal Metwally 🇪🇬
Endothelial cells (ECs) maintain about 60000 miles of blood vessels in human body. They are heterogenous group of cells that are responsive to signals from microenvironment. ECs line the blood and lymphatic vessels with exception of the placenta. These cells provide a physical barrier between the circulation and tissues.
EC activation is involved in the pathogenesis of severe malaria syndrome and networks of host pathways might be involved in the disease outcome. The parasite either relies on or dysregulates these pathways to survive in the human host. During P.falciparum infection, IEs are released into the blood circulation of the human host, and then the number of IEs increases exponentially until they trigger the host immune response. So far, innate immune responses triggered in response to malaria infection are described as the result of a rapture of schizonts, which leads to the release of large amounts of digestive vacuoles, hemozoin and waste products of the parasite in the circulation. Thus, the innate immune cells are stimulated and the production of proinflammatory responses is induced. Proinflammatory cytokines and chemokines activate ECs, which undergo changes resulting in “EC dysfunction”, typically characterized by decreased endothelial expression of nitric oxide (NO; a profound vasodilator), and increase the expression of cell adhesion molecules, resulting in increased binding of circulating leukocytes to these cells.
EVs derived from P. falciparum IEs are known to contain both parasite protein and miRNAs, which can be transferred to the recipient host and modulate target genes.
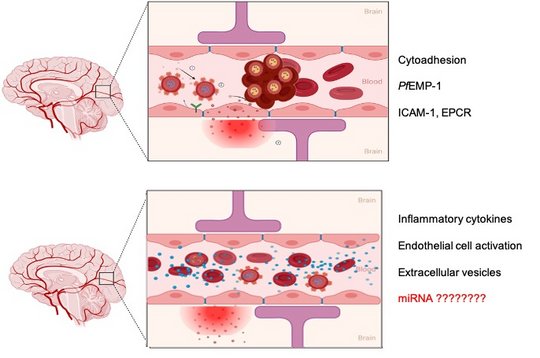
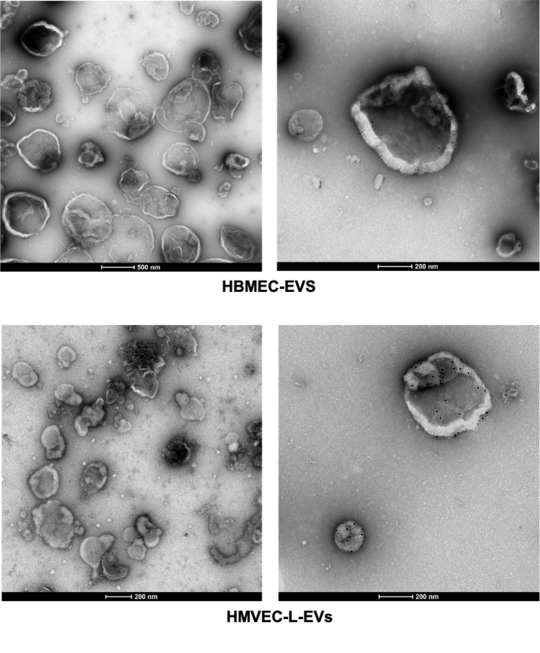
The role of EVs derived from human brain endothelial cells in the pathogenic cascade during severe malaria is not yet investigated.
In this project, we purify the EVs derived from primary brain endothelial cells and analysis thier miRNA profile under different stimulus.
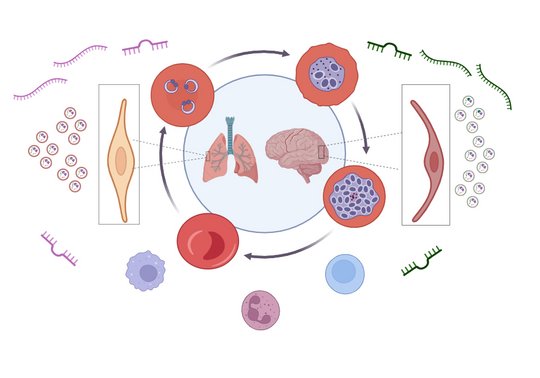
To dissect the distinct profiles of tissue specific-iRBCs communication, we designed this study to compare the miRNA/mRNA of primarylung ECs up on exposure to different stimuli that are present during P. falciparum infection. Aiming to deeply mine the transcriptomic data and piece together the puzzle of severe malaria.
Shear stress effect on the human endothelium
ECs are normally under different shear stresses depending on the dimension of vessels. Shear stress is defined as the dragging frictional force generated by flowing blood. ECs respond to shear stress through structural alignment of the cells and the secretion of subsets of proteins. Normal physiological flow is a laminar flow, associated with physiological phenotype of the ECs. The exposure of ECs to different shear stresses leads to various intracellular responses that modulated the expression of some receptors on the cell surface.
Authentic stimulation of the ECs is important to monitor in detail the first signal of the proinflammatory response and the following cascades. We, therefore, set out to examine the initiating signal for the activation of ECs during interaction with P. falciparum IEs (Wu and Bouws et al., 2021) (Metwally et al., 2024).

Human Blood brain barrier model under shear stress
Marlena Kemper 🇩🇪 Viktor Michaelis 🇩🇪 and Nahla Galal Metwally 🇪🇬
The BBB is a highly selective, semipermeable barrier which ensures the separation of circulating blood from the brain and the central nervous system (CNS). This important physiological barrier allows brain cells to fulfil their physiological function by regulating the delicate brain microenvironment and maintaining cerebral homeostasis in the CNS. Cerebrovascular ECs are the core anatomical component of the BBB and line the blood vessels of the vascular system of the CNS. Pericytes, supporting glial cells (astrocytes and microglia), basement membranes, and the extracellular matrix (ECM) also play vital roles in maintaining the integrity of the BBB. A malfunction of the BBB, linked to increased BBB permeability and dysregulated influx and efflux, results in toxins and immune cells infiltrating the CNS. A number of vascular events were identified to occur during severe malaria. The precise mechanism and sequence is unknown.
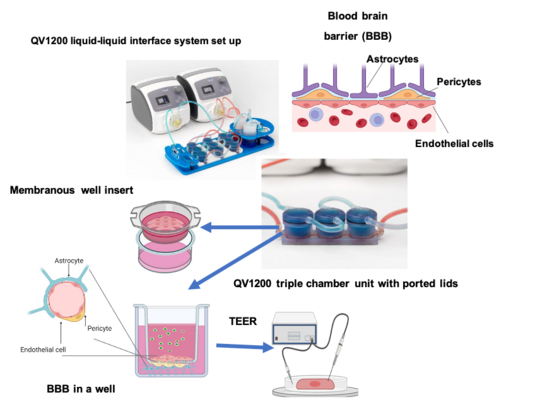
How human host cells react to / are affected by P. falciparum infection
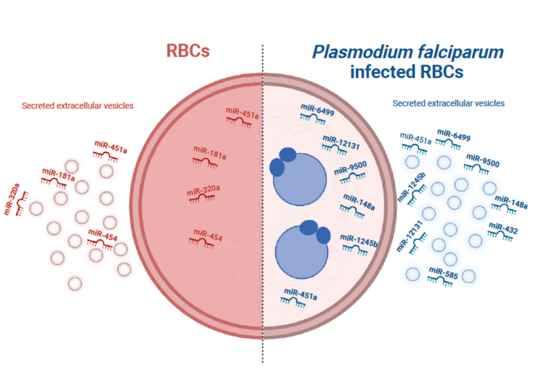
MiRNA profiles of human red blood cells
Yifan Wu 🇨🇳 , Nahla Galal Metwally 🇪🇬
Viable cells communicate indirectly by releasing extracellular vesicles (EVs) that contain miRNAs, mRNAs, and proteins. EVs are either formed inside multivesicular bodies (exosomes) or directly from the plasma membrane (microvesicles). EVs released from RBCs contain miRNAs coupled with Argonaute 2; these complexes can alter gene expression in other types of cells upon uptake of the RBC-EVs. Currently, minimal information is available on the role of miRNAs in the pathogenesis of P. falciparum complications.
We analyzed the miRNA profiles of non-infected human RBCs (niRBCs), ring-infected RBCs (riRBCs), and trophozoite-infected RBCs (trRBCs), as well as those of EVs secreted from these cells. Hsa-miR-451a was the most abundant miRNA in all RBC and RBC-EV populations, but its expression level was not affected by P. falciparum infection. Overall, the miRNA profiles of RBCs and their EVs were altered significantly after infection. Most of the differentially expressed miRNAs were shared between RBCs and their EVs. A target prediction analysis of the miRNAs revealed the possible identity of the genes targeted by these miRNAs (CXCL10, OAS1, IL7, and CCL5) involved in immunomodulation (Wu et al., 2023).
Functional analysis of these miRNAs will follow.
Alumni
Doctoral degree (PhD)
Maria del Pilar Martinez Tauler 🇪🇸 (2025)(Hamburg University): Characterisation of micro-RNA changes in human primary endothelial cells after exposure to Plasmodium falciparum (Welch, 1897) and their derived extracellular vesicles.
Yifan Wu 🇨🇳 (2023)(Hamburg University): The role of human endothelium and microRNAs as active participants in the immune response and pathogenesis during malaria
Doctoral degree (MD)
Tabea Schell 🇩🇪 (Hamburg University)(2024): The Influence of Cytoadhesion of P. falciparum Infected Erythrocytes on Human Lung Endothelial Cells
Master's degree
Nadja Götz 🇩🇪 (2025) (Johannes Gutenberg University Mainz): Characterisation of Extracellular Vesicles Secreted by HB3 Plasmodium falciparum-Infected Red Blood Cells that Bind to Human Brain and Lung Endothelial Cells
Maryéva Bessemoulin🇫🇷 (2023) (University of Strasbourg, France): Analysis of the activation of human brain endothelial cells during Plasmodium falciparum infection under shear stress
Hanifeh Torabi 🇮🇷 (2022)(Hamburg University): Influence of Plasmodium falciparum infected erythrocytes on the gene expression of human endothelial cells
Johannes Allweier 🇩🇪 (2021)(Hamburg University of Applied Science): Investigation of febrile conditions and stimulation through TNFa on human brain and lung endothelial cells under shear stress using Next-Generation-Sequencing
Shu 'Jerry' Njiyang 🇨🇲 (2020)(University of Lübeck): Characterising the response of different human endothelial cells exposed to cytoadhesion of P. falciparum infected erythrocytes
Jean Maximilian Rakotonirinalalao 🇩🇪 🇲🇬 (2020)(Hamburg University): Characterization of the binding capacity of Plasmodium falciparum infected erythrocytes to different endothelial cell lines
Philip Bouws 🇩🇪 (2019)(Hamburg University): Stimuli der knob-Ausbildung Plasmodium falciparum (WELCH, 1897) infizierter Erythrozyten
Bachelor degree
Fatima Alshikh 🇸🇾 (2024)(Hamburg University of Applied Science): Charakterisierung der Oberflächenmarker von menschlichen mikrovaskulären Hirnendothelzellen
Sara Mohamad 🇸🇾 (2024)(Hamburg University of Applied Science): Charakterisierung der Aktivierung von menschlichen Hirnendothelzellen nach der Co-inkubation mit HB3 Plasmodium falciparum unter Flussbedingungen
Margherita Pignataro 🇮🇹 (2023)(University of Eastern Piedmont – Italy): Analysing the binding capacity of P. falciparum infected erythrocytes expressing MAL6P1.252 gene to human brain endothelial cells.
Milad Temori 🇩🇪 🇦🇫 (2022)(Hamburg University of Applied Science): Anreicherung und Transkriptomanalyse von P. falciparum infizierten Erythrozyten, die an den humanen EPCR Rezeptor binden
David Danicic-Rauchberger🇦🇹(2021)(University of Applied Science - Austria): Characterization of the cytoadhesion of 3D7 Plasmodium falciparum infected erythrocytes expressiong MAL6P1.252 var gene to human brain and lung cells
Publications:
Leyk S, Liebold I, Lanzloth C, Richardt U, Kux JM, Kilian C, Moritz M, Gocke A, Meyer S, Höhn K, Honecker B, Haas H, Böttcher M, Weidemann S, Kind S, Schlüter H, Metwally NG, Adlung L, Sáez PJ, Jacobs T, Ruckdeschel K, Bosurgi L. Extracellular vesicle properties and functions are defined by the originating cell's fitness status. Journal of Extracellular Biology. 2026 doi: 10.1002/jex2.70115
Honecker B, Bärreiter VA, Höhn K, Horváth B, Harant K, Metwally NG, Marggraff C, Anders J, Leyk S, Martínez-Tauler MDP, Bea A, Hansen C, Fehling H, Lütkemeyer M, Lorenzen S, Franzenburg S, Lotter H, Bruchhaus I. Entamoeba histolytica extracellular vesicles drive pro-inflammatory monocyte signaling. PLoS Negl Trop Dis. 2025 Apr 10;19(4):e0012997. doi: 10.1371/journal.pntd.0012997. Epub ahead of print. PMID: 40208874.
Blancke Soares Alexandra, Stäcker Jan, Schwald Svenja, Hoijmakers Wieteke, Metwally Nahla Galal, Cronshagen Jakob, Schoeler Hanno, Flemming Sven, Höhn Katharina, Fröhlke Ulrike, Mesén-Ramírez Paolo, Bergmann Bärbel, Khosh-Naucke Melissa, Bruchhaus Iris, Bártfai Richárd, Spielmann Tobias (2025) An unusual trafficking domain in MSRP6 defines a complex needed for Maurer’s clefts anchoring and maintenance in P. falciparum infected red blood cells eLife 14:RP103633
Nahla Galal Metwally*, Maria del Pilar Martinez Tauler*, Hanifeh Torabi*, Johannes Allweier*, Sara Mohamed, Maryeva Bessemoulin, Philip Bouws, Fatima Alshikh, Yifan Wu, Milad Temori, Tabea Schell, Maximillian Rakotonirinalalao, Barbara Honecker, Katharina Höhn, Thomas Jacobs, Holger Heine, Iris Bruchhaus, Distinct brain and lung endothelial miRNA/mRNA profiles after exposure to Plasmodium falciparum-infected red blood cells, iScience, 2024, 111265, ISSN 2589-0042.
Darif ND, Ganter M, Dziekan JM, Kilian N, Brancucci N, Ng C, de Vries LE, Guttery D, Philip N, Boddey JA, Metwally NG, Okumu F, Kooij TWA, Absalon S, Bryant JM. BioMalPar XX: looking back on, and forward from, 20 years of malaria research. Trends Parasitol. 2024 Aug;40(8):651-656. doi: 10.1016/j.pt.2024.06.012. Epub 2024 Jul 15. PMID: 39013661.
Anders J, König C, Lender C, Hellhund A, Nehls S, Shalabi I, Honecker B, Lorenzen S, Meyer M, Matthiesen J, Cadar D, Roeder T, Galal Metwally N, Lotter H, Bruchhaus I. Genes differentially expressed between pathogenic and non-pathogenic Entamoeba histolytica clones influence pathogenicity-associated phenotypes by multiple mechanisms. PLoS Pathog. 2023 Dec 22;19(12):e1011745. doi: 10.1371/journal.ppat.1011745. PMID: 38134215; PMCID: PMC10773965.
Wu Y, Leyk S, Torabi H, Höhn K, Honecker B, Tauler M, Cadar D, Jacobs T, Bruchhaus I, and Metwally NG. 2023 “Plasmodium falciparum infection reshapes the human microRNA profiles of red blood cells and their extracellular vesicles” 2023 June 15. doi.org/10.1016/j.isci.2023.107119.
Bachmann A*, Metwally NG*, Allweier J, Cronshagen J, Del Pilar Martinez Tauler M, Murk A, Roth LK, Torabi H, Wu Y, Gutsmann T, Bruchhaus I. CD36-A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms. 2022 Nov 29;10(12):2356. doi: 10.3390/microorganisms10122356. PMID: 36557610; *contributed equally.
Rehn T, Lubiana P, Nguyen THT, Pansegrau E, Schmitt M, Roth LK, Brehmer J, Roeder T, Cadar D, Metwally NG, Bruchhaus I. Ectopic Expression of Plasmodium vivax vir Genes in P. falciparum Affects Cytoadhesion via Increased Expression of Specific var Genes. Microorganisms. 2022 Jun 9;10(6):1183. doi: 10.3390/microorganisms10061183. PMID: 35744701.
Groneberg M, Hoenow S, Marggraff C, Fehling H, Metwally NG, Hansen C, Bruchhaus I, Tiegs G, Sellau J, Lotter H. HIF-1α modulates sex-specific Th17/Treg responses during hepatic amoebiasis. J Hepatol. 2022 Jan;76(1):160-173. doi: 10.1016/j.jhep.2021.09.020. Epub 2021 Sep 29. PMID: 34599999.
Raacke M, Kerr A, Dörpinghaus M, Brehmer J, Wu Y, Lorenzen S, Fink C, Jacobs T, Roeder T, Sellau J, Bachmann A, Metwally NG, Bruchhaus I. Altered Cytokine Response of Human Brain Endothelial Cells after Stimulation with Malaria Patient Plasma. Cells. 2021 Jul 1;10(7):1656. doi: 10.3390/cells10071656. PMID: 34359826
Wu Y, Bouws P, Lorenzen S, Bruchhaus I, Metwally NG. Analysis of the Interaction Between Plasmodium falciparum-Infected Erythrocytes and Human Endothelial Cells Using a Laminar Flow System, Bioinformatic Tracking and Transcriptome Analysis. Methods Mol Biol. 2021;2369:187-197. doi: 10.1007/978-1-0716-1681-9_11. PMID: 34313990.
König C, Honecker B, Wilson IW, Weedall GD, Hall N, Roeder T, Metwally NG, Bruchhaus I. Taxon-Specific Proteins of the Pathogenic Entamoeba Species E. histolytica and E. nuttalli. Front Cell Infect Microbiol. 2021 Mar 19;11:641472. doi: 10.3389/fcimb.2021.641472. PMID: 33816346; PMCID: PMC8017271.
König C, Meyer M, Lender C, Nehls S, Wallaschkowski T, Holm T, Matthies T, Lercher D, Matthiesen J, Fehling H, Roeder T, Reindl S, Rosenthal M, Metwally NG, Lotter H, Bruchhaus I. An Alcohol Dehydrogenase 3 (ADH3) from Entamoeba histolytica Is Involved in the Detoxification of Toxic Aldehydes. Microorganisms. 2020 Oct 19;8(10):1608. doi: 10.3390/microorganisms8101608. PMID: 33086693; PMCID: PMC7594077.
Sellau J, Groneberg M, Fehling H, Thye T, Hoenow S, Marggraff C, Weskamm M, Hansen C, Stanelle-Bertram S, Kuehl S, Noll J, Wolf V, Metwally NG, Hagen SH, Dorn C, Wernecke J, Ittrich H, Tannich E, Jacobs T, Bruchhaus I, Altfeld M, Lotter H. Androgens predispose males to monocyte-mediated immunopathology by inducing the expression of leukocyte recruitment factor CXCL1. Nat Commun. 2020 Jul 10;11(1):3459. doi: 10.1038/s41467-020-17260-y. PMID: 32651360; PMCID: PMC7351718.
Lubiana P, Bouws P, Roth LK, Dörpinghaus M, Rehn T, Brehmer J, Wichers JS, Bachmann A, Höhn K, Roeder T, Thye T, Gutsmann T, Burmester T, Bruchhaus I, Metwally NG. Adhesion between P. falciparum infected erythrocytes and human endothelial receptors follows alternative binding dynamics under flow and febrile conditions. Sci Rep. 2020 Mar 11;10(1):4548. doi: 10.1038/s41598-020-61388-2. PMID: 32161335; PMCID: PMC7066226.
Dörpinghaus M, Fürstenwerth F, Roth LK, Bouws P, Rakotonirinalalao M, Jordan V, Sauer M, Rehn T, Pansegrau E, Höhn K, Mesén-Ramírez P, Bachmann A, Lorenzen S, Roeder T, Metwally NG, Bruchhaus I. Stringent Selection of Knobby Plasmodium falciparum-Infected Erythrocytes during Cytoadhesion at Febrile Temperature. Microorganisms. 2020 Jan 25;8(2):174. doi: 10.3390/microorganisms8020174. PMID: 31991814; PMCID: PMC7074740.
Metwally NG, Tilly AK, Lubiana P, Roth LK, Dörpinghaus M, Lorenzen S, Schuldt K, Witt S, Bachmann A, Tidow H, Gutsmann T, Burmester T, Roeder T, Tannich E, Bruchhaus I. Characterisation of Plasmodium falciparum populations selected on the human endothelial receptors P-selectin, E-selectin, CD9 and CD151. Sci Rep. 2017 Jun 22;7(1):4069. doi: 10.1038/s41598-017-04241-3. PMID: 28642573; PMCID: PMC5481354.
Tilly AK, Thiede J, Metwally N, Lubiana P, Bachmann A, Roeder T, Rockliffe N, Lorenzen S, Tannich E, Gutsmann T, Bruchhaus I. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci Rep. 2015 Nov 16;5:16766. doi: 10.1038/srep16766. PMID: 26568166; PMCID: PMC4645185.
Rayan HZ, Soliman RH, Galal, NM (2012). Detection of Strongyloides stercoralis in Fecal Samples Using Conventional Parasitological Techniques and Real-Time PCR: A Comparative Study. PUJ Volume (5:1).