Our research projects at a glance
Viral fitness refers to the relative ability of a virus to survive, replicate, and spread within a specific host population or environment. Viruses carrying traits that confer a competitive advantage, such as those that improve replication efficiency or evasion of host immune responses, are favored over time, and critical contributing features are maintained in subsequent generations during evolution. Common fitness elements within related viruses can include conserved regions in the viral genome crucial for replication, key protein domains or motifs involved in host interaction or immune evasion, and common pathways or mechanisms utilized by viruses within the family for replication and transmission. Thus, targeting shared viral fitness elements can aid in developing medical countermeasures with a broad scope of action against infections from emerging and re-emerging human pathogens prone to cause public health emergencies of international concern.
Most recently, our work has shown the biological relevance of potentially druggable pockets newly identified by X-ray screening on SARS-CoV-2 (family Coronaviridae) non-structural proteins such as Nsp5, also known as MPro, and complexed Nsp10/Nsp16.
Mpro is the main protease participating in the maturation process of the virus's functional proteins. Its enzymatic activity relies on the architecture of the active site, which critically depends on the dimerization of the enzyme. Accordingly, allosteric regulation of the proteolytic cleavage reaction by small molecules can be a valid way to design intervention therapies. Interestingly, exposed allosteric site 1 (depicted in the figure below) is located at the C-terminal of the Mpro dimerization domain and possesses a hydrophobic cavity conserved in all homologous proteins of human coronaviruses. Although not as potent as the effect of active site peptidomimetic inhibitor calpeptin, the reduction of viral RNA and infectious particles of SARS-CoV-2 in the supernatant of VeroE6 cells treated with the allosteric site 1 binder pelitinib suggests a plausible antiviral inhibition through this hot spot.
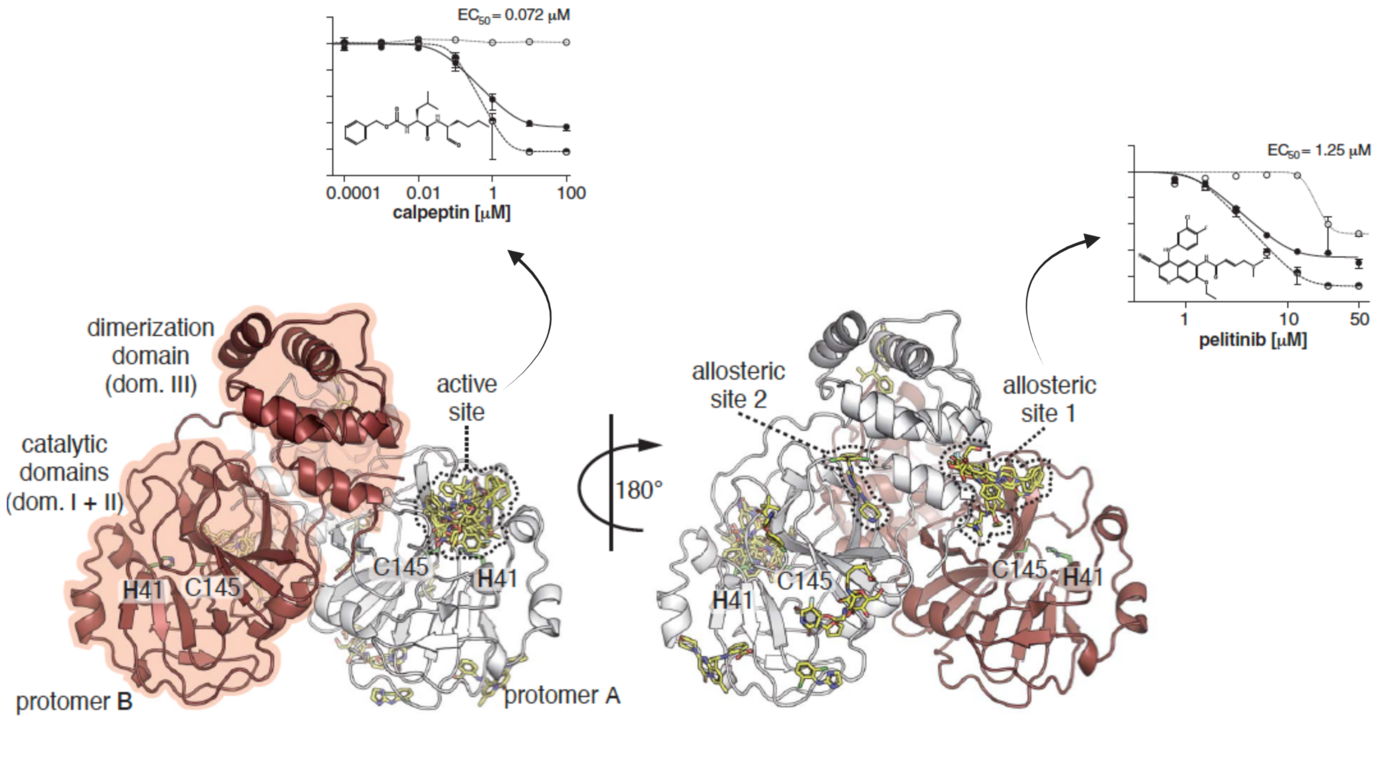
Science. 372(6542): 642-646. doi:10.1126/science.abf7945. @Science
Like SARS-CoV-2, one of the most deadly organisms in the Americas with case fatality rates of up to roughly 40% and human-to-human transmission with documented super-spreader events is another airborne respiratory virus known as Andes virus (ANDV; family Hantaviridae). We have solved the tridimensional structure and biochemically characterized a metal-dependent endoribonuclease domain (illustrated in the figure on the right) at the N-terminal site of the virus L protein. This enzyme is responsible for initiating the virus's genome transcription via a cap-snatching mechanism, making it a driving factor in determining the magnitude of viral amplification and, therefore, the progression of the infectious disease. Its vital role in the early stages of the virus lifecycle and uniqueness to segmented single-stranded RNA viruses defines it as a strategic pharmacological target.
Proof-of-concept for the clinical applicability of cap-snatching endonuclease (Cap-ENDO) inhibitors is the recently approved antiviral Xofluza. Baloxavir acid, the active compound in Xofluza, targets the endonuclease of the Influenza virus. The drug, administered as a single-dose regimen, has proven effective against circulating strains resistant to current treatments.
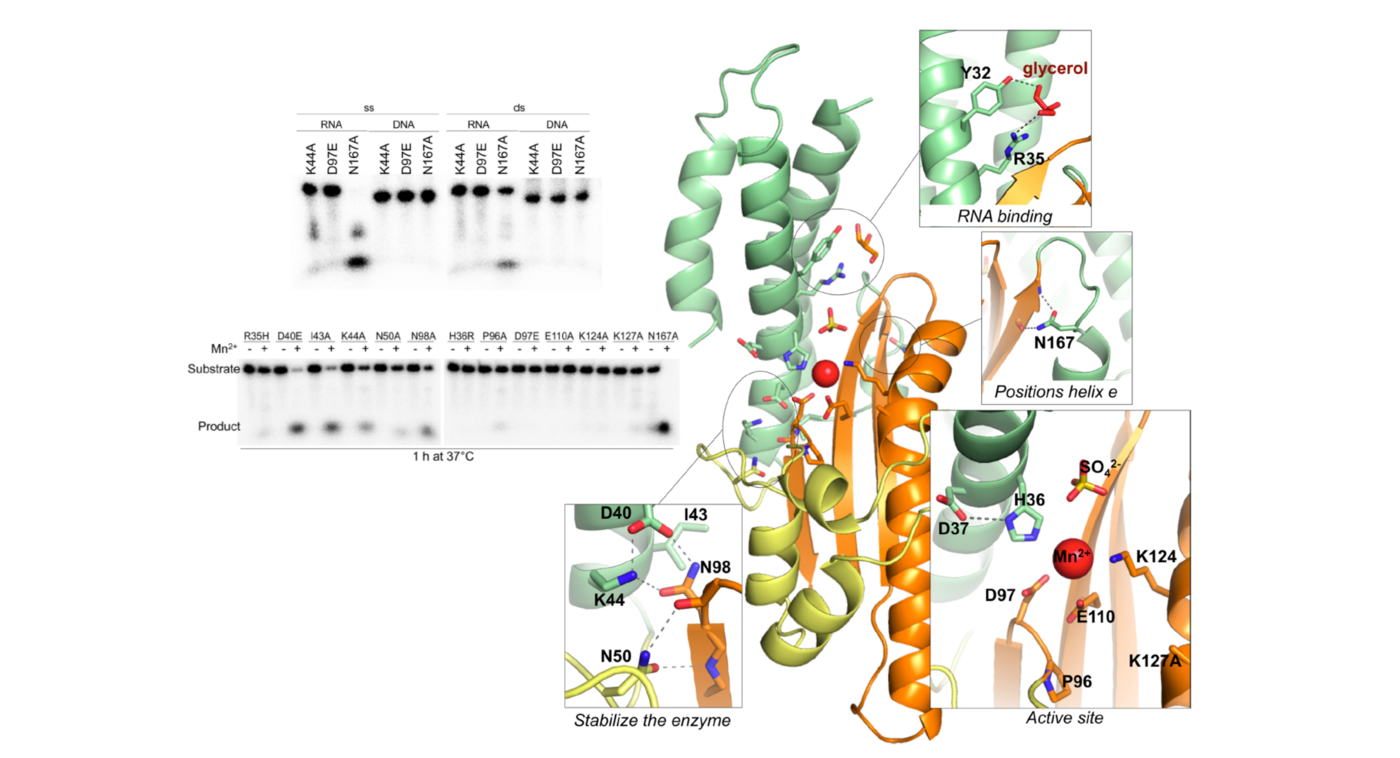
PLoS Pathogens. 12(6): e1005635. doi:10.1371/journal.ppat.1005635. @PLoS Pathogens
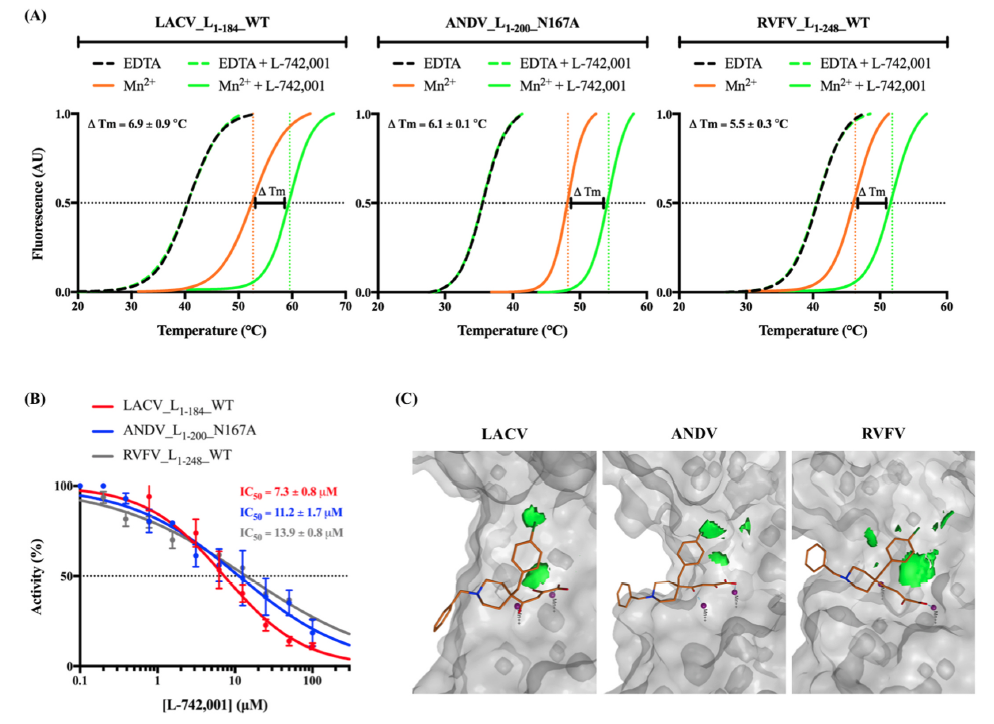
Although the specifics of the mechanism might vary between bunyaviruses (e.g., families Hantaviridae, Peribunyaviridae, Phenuiviridae, Arenaviridae), a constant feature in their genome transcription is the Cap-ENDO responsible for the host cellular mRNA cleavage. Integrating the results obtained from biophysical and biochemical methods together with the structural analysis of predicted interactions, we have identified conserved traits of both the viral target and a class of metal chelators that hint at the possibility of developing pan-bunyavirus therapeutics (presented in the image below). However, little is known about the ligand-reactive landscape of Bunyavirales Cap-ENDOs. We seek to reduce this knowledge gap by mapping hot spot terrains of homologous Cap-ENDOs from diverse human pathogens.
Antiviral Research. 183: 104947. doi:10.1016/j.antiviral.2020.104947. @PLoS Pathogens













