Follow-up Study of Lassa Fever Survivors
Awareness of Lassa fever (LF) as a public health threat is increasing and it is listed as priority disease in the WHO R&D blueprint. Currently, six Lassa virus (LASV)-vaccine candidates are investigated in clinical trials and knowledge about natural long-term immunity after an infection is urgently needed to assess vaccine efficacy. Data about LASV-specific antibodies as a correlate of protection, however, are scarce.
Furthermore, only little is known about viral persistence after a LASV infection. For Ebola virus it is shown that infectious virus can persist especially in semen of male survivors for over 2 years after recovery. Such knowledge is critical to improve public health measures to prevent transmission, but also for drug development, as persistence in immune-privileged sides needs to be taken into account.
To fill these knowledge gaps, a prospective longitudinal cohort study to follow-up LF survivors after discharge from the isolation ward of the Irrua Specialist Teaching Hospital in Edo state, Nigeria is performed. The study contains two cohorts, which are both followed for 5 years.
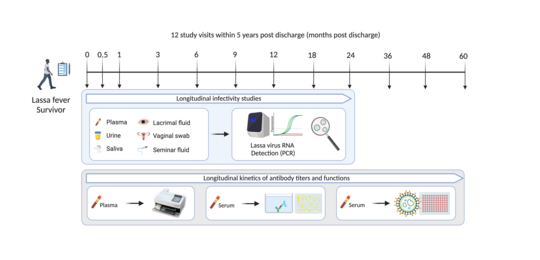
In cohort 1, 157 participants have been enrolled in 2018 and 2019. During their follow up 12 visits are scheduled. To characterize the humoral immune response against a natural Lassa virus infection, the longitudinal kinetics of antibody levels against different LASV antigens is investigated. Further antibody functions are examined in addition. This knowledge improves the basic understanding of the interaction between LASV and the virus-specific long-term immune response. To investigate potential persistence of LASV in various body fluids, urine, saliva, lacrimal fluid, and a vaginal swab or seminal fluid are collected within the first 2 years of follow up. The presence of LASV RNA is determined by real-time RT-PCR and positive specimen are used to perform infectivity studies.
The enrollment of cohort 2 started in January 2022 and is still ongoing. The focus of this cohort is the investigation of the longitudinal immune response with a focus on the cellular immunity, and more specific, on the T-cell response.



![[Translate to English:] [Translate to English:] Logo DFG](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DFG.png)
![[Translate to English:] [Translate to English:] Logo DZIF](/fileadmin/media/Das_Institut/Kooperationen/Logo_DZIF_01.png)
![[Translate to English:] [Translate to English:] Logo Leibniz Gemeinschaft](/fileadmin/media/Das_Institut/Kooperationen/Logo_Leibniz_Gemeinschaft.png)
![[Translate to English:] [Translate to English:] Logo Jürgen Manchot Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Juergen_Manchot_Stiftung.png)





