Arbovirus-host interaction
Mosquito-borne viruses are important human and animal pathogens with some of them having a high zoonosis potential, like Rift valley fever virus, West Nile virus and Usutuvirus. Understanding the interaction of these viruses with the vertebrate host is important to (i) determine the risk of zoonotic viruses to be transmitted from human to mosquito to human, (ii) finding new targets for antivirals, (iii) determining new possible vaccine candidates and (iv) be able to predict such infections in humans and their pathogenicity. In the past, for most virus-host interaction studies, mosquito-borne viruses have been injected into the animal model often using viruses produced in mammalian cells.
In recent years, results have highlighted differences in the infection and pathogenicity of arboviruses, in their mammalian hosts, depending on the cells used for virus production as well as the influence of mosquito factors, like saliva. Understanding the immune response and pathogenicity of arboviruses under “natural transmission” is important for vaccine and antiviral tests. Comparing the response to other transmission routes, broadens the understanding of virus- host interaction and to identify specific targets for intervention strategies.
Moreover, changes in the arbovirus genome/ proteins have been reported depending on the organisms used for virus amplification. However little is known about the effects of these genome changes on arbovirus replication and infection of its various host and vectors.
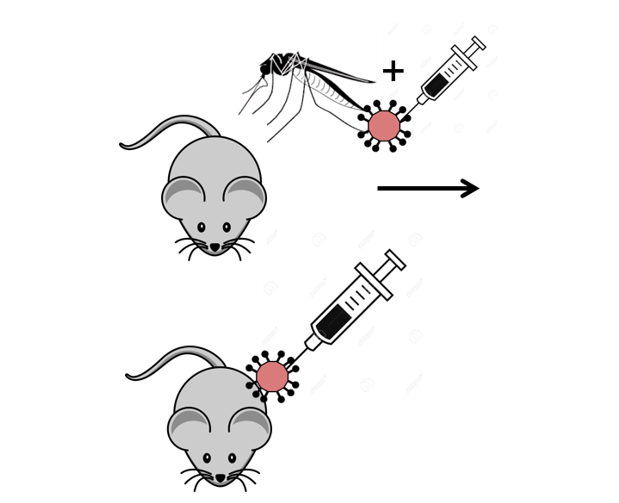
The importance of the natural transmission of Rift Valley Fever Virus for its infection in the mammalian host (DZIF, Joachim Herz-Stiftung)
Rift Valley Fever Virus (RVFV) is a Phlebovirus, transmitted mostly by Aedes and Culex mosquitoes in nature. The virus shows high mortality in several domesticated farm animals and it can cause severe, sometimes lethal infections in humans. Human infections may occur after a bite of an infected mosquito or after direct contact with viral particles on the skin or on mucosal surfaces. In this study we aimed to understand how factors, such as the infection site, the virus origin and mosquito factors like the saliva, may influence the pathogenesis of RVFV in mice.
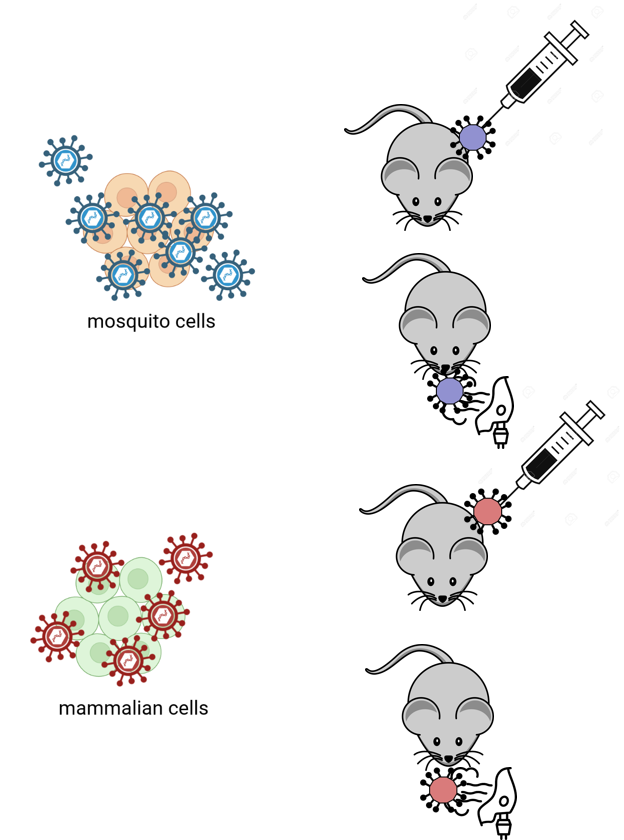
Adaptation, transmission and infection of West Nile virus in the mammalian host (INI Research)
West Nile virus (WNV) is known to replicate in a variety of organisms, including mosquitoes, birds and humans. Therefore, it can be expected that WNV adapts at least partly to the different organisms. However what effects this has on the corresponding WNV infection in a different host or vector is not known.
WNV does not cause any symptoms in the majority of infected humans; however a small number gets ill and often dies from the WNV infection (mainly due to encephalitis). How the virus causes encephalitis and why some virus strains are more pathogenic to others is not yet known, neither the effect of mosquito factors on the arbovirus infection.
Development of a live-attenuated vaccine candidate against Chikungunya virus (DZIF maternity grant)
With the establishment of Aedes albopictus populations in the Upper Rhine Valley, the risk of autochthonous acquired Chikungunya virus (CHIKV) infections in Germany is increasing. Until today, vector control is the only control tool available, since no CHIKV vaccine has been licensed yet.
A novel method of attenuation for viral vaccine development is micro(mi)RNA targeting. Viral replication can be strategically attenuated in specific organs or species. An attractive variation of this method for the development of vaccines against CHIKV is the dual-targeting of mammalian and mosquito miRNA. This way, accidental virus replication in the vector mosquito and reversal to wild-type in nature is prevented, while simultaneously attenuating the virus in the mammalian host. This approach is also applicable for other mosquito-borne viruses of medical and veterinary concern and is therefore an important step to reduce the burden of arboviral disease.
This project will analyze CHIKV live-attenuated vaccine (LAV) candidate(s) in cell culture as well as in vivo in the mosquito vector. The final aim is to identify the CHIKV LAV with greatest potential to be transferred to further testing in a mouse model.
The attenuation will be achieved mainly via a miRNA-targeting approach aiming to selectively restrict replication in neuronal cells and the mosquito vector. First, reporter (luciferase) plasmids containing miRNA-target-cassettes will be used for proof-of-concept analysis in vitro. Eventually, replication capability and genetic stability of candidate CHIKV LAVs will be determined in mammalian and insect cell lines via replication assays and passaging. Lastly, the vector competence of mosquito vectors for promising candidates will be investigated.


![[Translate to English:] Logo Zikalliance](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_ZIKAlliance.jpg)
![Logo Deutsches Zentrum für Infektionsforschung (DZIF) [Translate to English:] Logo DZIF](/fileadmin/media/Das_Institut/Kooperationen/Logo_DZIF_01.png)
![[Translate to English:] Logo Daad](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DAAD.png)
![[Translate to English:] Logo Joachim Herz Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Joachim_Herz_Stiftung.jpg)
![[Translate to English:] [Translate to English:] Logo DFG: Zu sehen ist der Schriftzu DFG in dicker blauer Schrift.](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DFG.png)
![[Translate to English:] [Translate to English:] Logo Bundesministerium für Ernährung und Landwirtschaft: zu sehen ist der schwarze Bundesadler, daneben ist die deutsche Flagge in einem dünnen Strich angedeutet (schwarz, rot Gold). Auf der rechten Seite steht in schwarz der Schriftzug Bundesministerium für Ernährung und Landwirtschaft.](/fileadmin/media/Forschung/forschungsgruppen/Population/Abteilung_Arbovirologie_und_Entomologie/AG_Virus_Metagenomik_und_Evolution/BMEL_Logo.svg.png)





