Autoimmunity protects against malaria
Understanding the interaction between infectious disease and autoimmunity
Malaria is one of the world's leading infectious diseases. It leads to death in many children. Researchers at the Bernhard Nocht Institute for Tropical Medicine (BNITM), together with collaboration partners, have discovered that autoimmunity associates with protection against clinical malaria. The study shows that autoantibodies inhibit the growth of the malaria parasite and bind to proteins that are important for invasion of red blood cells. The study was recently published in the journal Immunity.
Malaria has been around for at least a hundred thousand years. It has accompanied humans throughout their evolution. The single-celled malaria parasite Plasmodium falciparum causes the severe form of the disease, malaria tropica. In 2022, an estimated 250 million people contracted malaria, the majority of them malaria tropica; 600,000 of them died, most of them children.
Autoimmunity protects against malaria
Autoimmunity is an immune response directed against the body's own structures and can lead to autoimmune diseases such as rheumatism - autoantibodies bind to the body's own tissues. There is a known link between autoimmunity and malaria: Many previous studies have found autoantibodies in malaria patients. What these studies have in common is that the autoantibodies were measured after the malaria infection.

“It was known that malaria disease triggers the formation of autoantibodies,” says Dr Christine Hopp, author of the study and head of a laboratory group at the BNITM. “We wanted to test whether autoantibodies detectable before malaria in humans protect against febrile malaria.”
Hopp started the study while she was a postdoctoral fellow at the National Institute of Allergy and Infectious Diseases in the US. Colleagues at the Malaria Research and Training Center in Bamako, Mali, conducted the cohort study in Mali.
The researchers measured the levels of autoantibodies in 602 healthy children and adults in Mali before the malaria season and observed which of these people developed malaria in the following months. They found that people with high levels of autoantibodies had a 40% lower risk of developing a febrile malaria infection. The research team used statistical methods to check that other factors, such as age, did not distort the results. "It is the autoantibodies that are associated with the protection against febrile malaria infection," concludes Hopp.
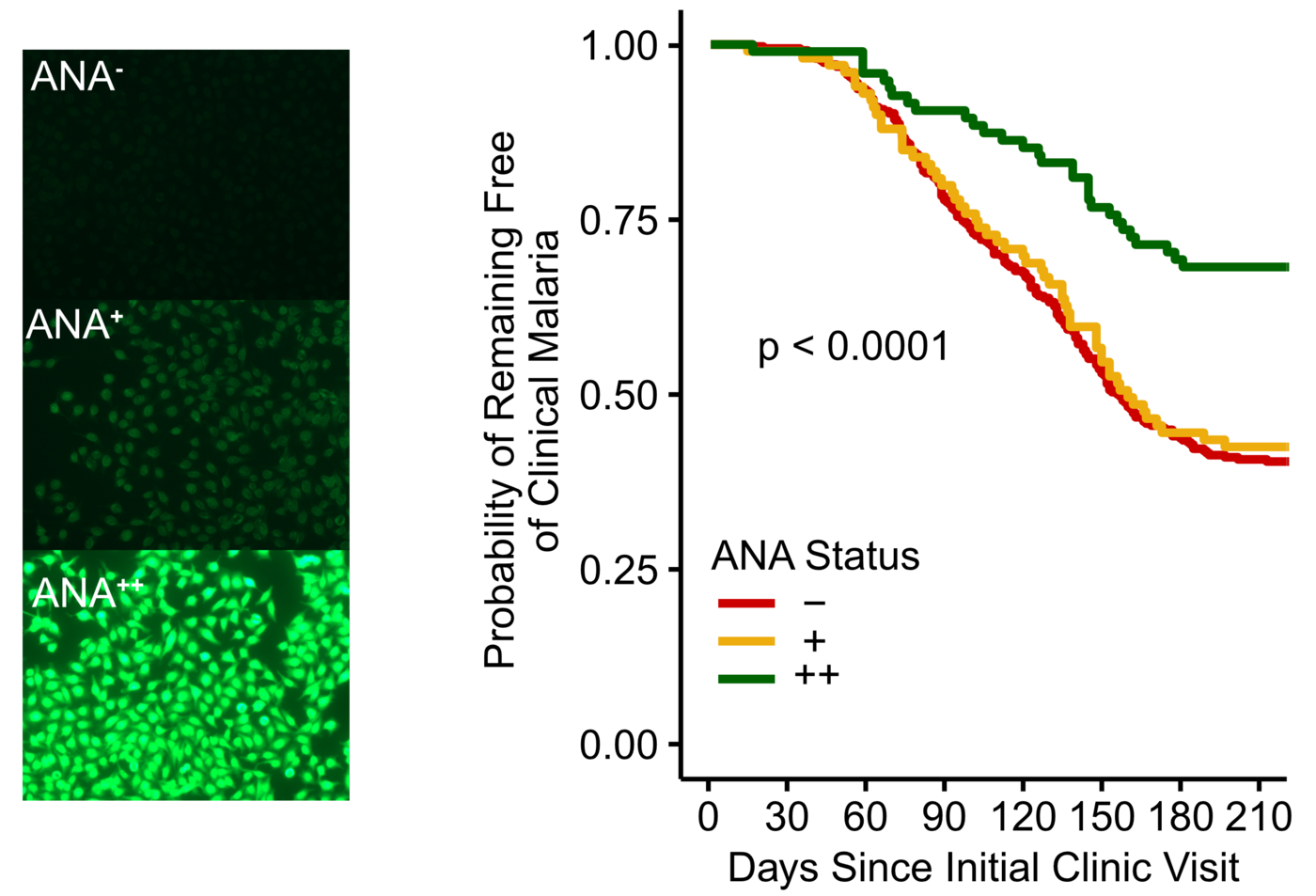
Autoantibodies inhibit the growth of the malaria parasite and bind to proteins that are key for parasite invasion of red blood cells
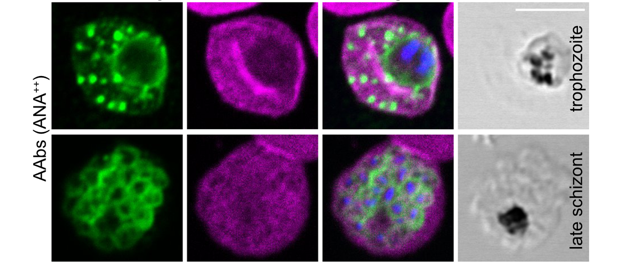
Hopp and her collaborators also carried out experiments with parasite cultures to investigate the mechanism by which autoantibodies can protect against infection with the malaria parasite Plasmodium falciparum. They cultured red blood cells with the parasite. The parasite enters the blood cells, matures, multiplies, the blood cells burst and the newly formed parasites infect other red blood cells. When the scientists added autoantibodies, the parasites grew less. But why? The researchers showed that the autoantibodies bind to the parasites in the infected blood cells. To find out more about where the autoantibodies bind to the parasite, they carried out binding studies. The autoantibodies bound to some of the parasite's proteins that are important for entering the blood cell.
Crucial to the cell culture experiments and binding studies was that Hopp and her colleagues had developed a method to isolate the autoantibodies from the other antibodies in the blood plasma samples of the volunteers.
Selective pressure of malaria on human genes
"What is exciting about this study is that it gives us an insight into the co-evolution of humans and malaria," says Hopp. Our immune system is faced with the difficult task of recognising pathogens as foreign structures on the one hand, and the body's own tissues and molecules as endogenous structures on the other. If this differentiation is successful, an immune response will only occur against foreign structures such as pathogens. How do pathogens evade the immune response? One mechanism used by pathogens is to resemble the body's own structures in such a way that the body does not recognise them as foreign. This is known as molecular mimicry. It seems likely that in its long co-evolution with humans, the malaria parasite has become increasingly similar to the body's own structures. As a counter-evolution, our immune system has adapted: It allows for more autoimmunity, because in this way it is able to provide a better immune response targeting the parasite.
African Americans in the US are more susceptible to autoimmune diseases than people of European descent. “Our new data suggest that this higher susceptibility to autoimmune disease may have arisen long ago in Africa, because there is a survival advantage to malaria when there is a propensity for autoimmunity. Our results suggest that malaria may have been a strong driver for the emergence of autoimmunity,” explains Hopp.
Original publication
Cooperation partner institutions
- National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH), Rockville, MD, USA
- Yale School of Public Health, Department of Epidemiology of Microbial Diseases, New Haven, CT, USA
- Division of Malaria Research, Proteo-Science Center, Ehime University, Matsuyama, Japan
- Malaria Research and Training Centre, Department of Epidemiology of Parasitic Diseases, International Center of Excellence in Research, University of Sciences, Technique and Technology of Bamako, Mali
- Department of Immunology and Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
- Genecopoeia Inc, Rockville, MD, USA
- Centre for Malaria Elimination, Institute of Tropical Medicine, Mount Kenya University, Thika, Kenya
- Department of Medicine, Division of Rheumatology, Lowance Center for Human Immunology and Emory Autoimmunity Center of Excellence, Emory University, Atlanta, GA, USA
- Division of Cell-Free Sciences, Proteo-Science Center, Ehime University, Matsuyama, Japan
Funding
Contact person
Dr Christine Hopp
Principal Investigator
Email : Christine.Hopp@bnitm.de
Dr Anna Hein
Public Relations
Phone : +49 40 285380-269
Email : presse@bnitm.de
Further information






