Caught red-handed
Researchers observe how a phenuivirus steals host mRNA caps
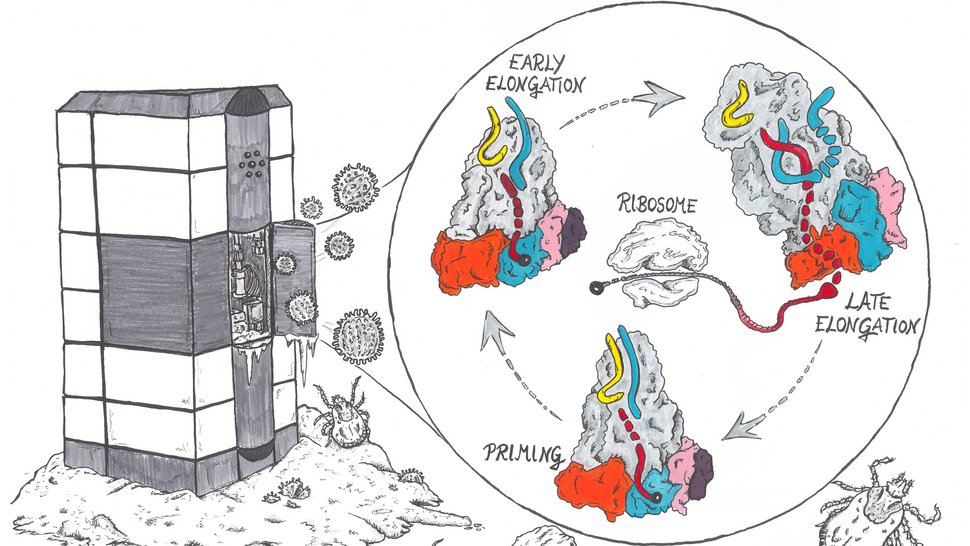
Viruses use the molecular infrastructure of infected host cells for their reproduction. While the viral polymerase replicates the genetic information of the virus, the host ribosome is needed to produce the viral proteins. However, the process by which a ribosome converts RNA into a protein – called translation – is tightly controlled and requires a so-called 5'RNA cap. Phenuiviruses cannot produce these RNA caps themselves, but steal them from their host. But how does this work? Researchers at the Bernhard Nocht Institute for Tropical Medicine (BNITM) and their collaborators have used cryo-electron microscopy to study and visualise different stages of this process on a molecular level. The results were published in the scientific journal Nucleic Acids Research.
Severe fever with thrombocytopenia syndrome virus (SFTS virus) is an RNA phenuivirus belonging to the group of Bunyaviruses. It causes an illness in humans that includes fever, muscle aches, diarrhea and a drop in blood platelets (thrombocytes). In severe cases, internal bleeding and multiple organ failure can also occur. Ticks transmit the SFTS virus and can infect a range of pets and livestock, including cats and cows, as well as humans. There are currently no effective drugs approved for use against the SFTS virus.
Hijacking the host protein factory
“Bunyaviruses need a kind of small key to gain access to the host cell's machinery,” explains Dr Maria Rosenthal, group leader of the BMBF junior research group Structural Virology at the BNITM. “These keys are the host's RNA caps. Viruses use this key to hijack the ribosome, which is the host's protein production factory.”

This is how proteins are made in the host cell: The ribosome recognises the RNA cap of a messenger RNA (mRNA) and begins to produce proteins. If there is no RNA cap on an RNA strand, usually no protein is made. Bunyavirus RNA does not contain such a cap. These viruses therefore need a strategy to get hold of the caps – i.e. the key. Some viruses, including SFTS virus, steal the RNA caps from the host cell by cutting them off the host RNA. The L protein of the SFTS virus was expected to be involved in this process. But how the process worked in detail was not known. In this study, researchers used cryo-electron microscopy (cryo-EM) to gain more insight into this process.

Structure determination by cryo-electron microscopy
First author Dr Harry M. Williams and his colleagues at the cryo-EM facility at the Centre for Structural Systems Biology (CSSB), led by Prof. Dr Kay Grünewald, used cryo-EM to take a series of images of the L protein. A single image is not enough to resolve the three-dimensional structure of the SFTS virus L protein for several reasons. Proteins are three-dimensional objects, so looking at the L protein from only direction would give the researchers only a partial understanding of its overall structure.
“Another limitation is that the contrast of images taken during cryo-EM experiments is typically very poor. To overcome this, we take many images of the L proteins during a single experiment. We then superimpose these images and subtract the background signal. However, we have to be careful that we only sum together images showing the L protein in similar shape. The L protein is large and has flexible domains that can point in different directions. So we often spend a lot of time trying to work out which images we can combine,” says Williams, describing the process.
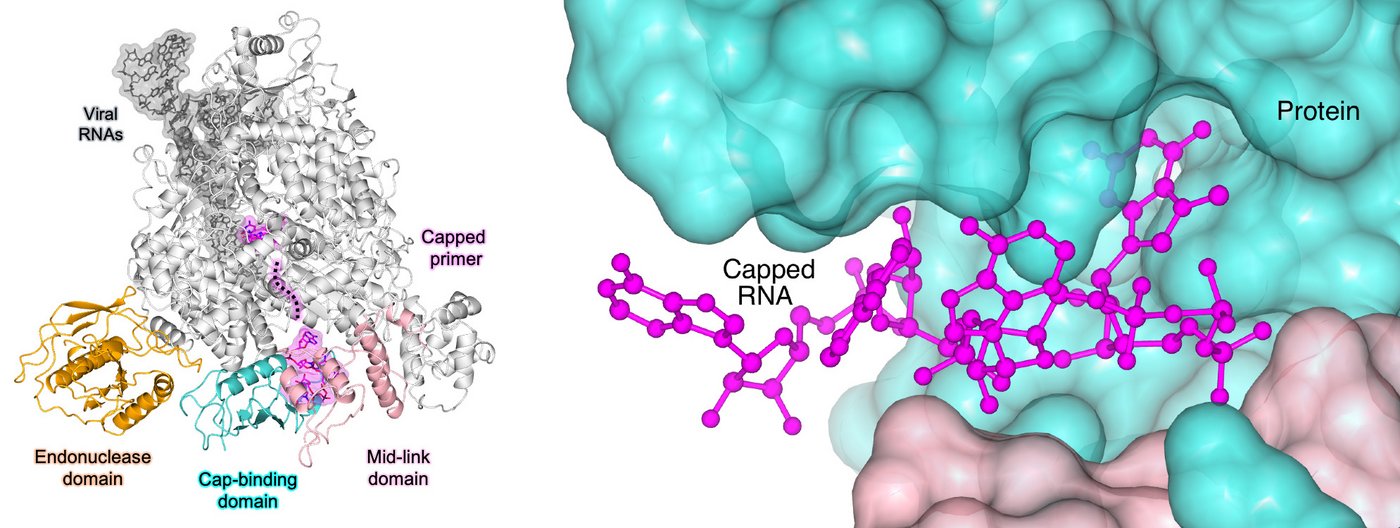
Viral L protein steals host RNA cap
Using the structural insights gained from cryo-EM and additional experiments, the research team was able to show how the viral L protein of the SFTS virus binds to host RNA caps and could cleave them from the rest of the RNA strand. They also showed that the virus uses the RNA caps as a starting point for the formation of a new viral mRNA strand. “While we think there certainly will be a role for host cell factors in perhaps delivering or handing off host mRNA to these L proteins, the process of cap-snatching itself is entirely driven by the L protein. The L protein alone is sufficient to recognise the host RNA, cut off the cap and synthesise new viral mRNA,” says Williams. In the next step, which the researchers have not yet investigated in this study, the host cell ribosome recognises the RNA cap on the newly formed viral mRNA strand and translates it into viral proteins.
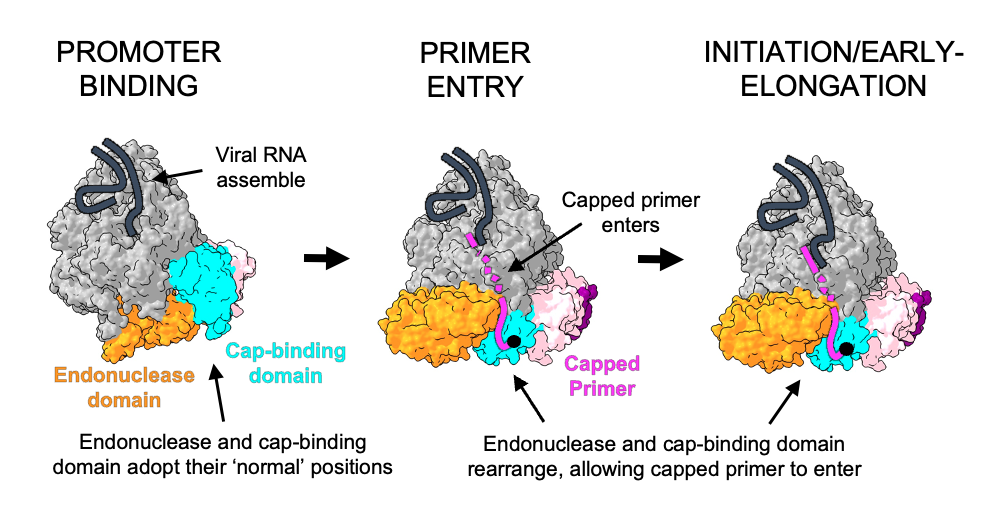
Cap-snatching as a target for new drugs
Key steps in the life cycle of viruses can be targets for the development of inhibitory drugs. Researchers use structure determination methods such as cryo-EM to identify sites in the viral proteins where drugs can act. “If we could specifically inhibit the process of viral transcription, i.e. the formation of a new viral mRNA strand for protein synthesis, the life cycle of the virus would be interrupted," concludes group leader Rosenthal. In current and future studies, Rosenthal and her team are trying to find antiviral agents targeting virus-specific processes conducted by the L protein and including the described cap-snatching mechanism.
Original publication
Cooperation partner institutions
- Centre for Structural Systems Biology (CSSB), Hamburg, Germany
- Leibniz Institute of Virology, Hamburg, Germany
- University of Hamburg, Hamburg, Germany
- European Molecular Biology Laboratory, Grenoble, France
- Department of Virology, Institute for Integrative Biology of the Cell (I2BC), Centre National de la Recherche Scientifique (CNRS) UMR9198,Gif-sur-Yvette, France
- Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Discovery Research ScreeningPort, Hamburg, Germany
Funding
Contact person
Dr Maria Rosenthal
Research Group Leader
Phone : +49 40 285380-930
Email : rosenthal@bnitm.de
PhD Harry Williams
Phone : +49 40 285380-930
Email : harry.williams@bnitm.de
Dr Anna Hein
Public Relations
Phone : +49 40 285380-269
Email : presse@bnitm.de
Further information







