Infectious master thieves or how malaria parasites steal and digest our red blood pigment
It is a sophisticated molecular mechanism by which malaria parasites take up the contents of their host cells into their own cells and break them down to survive. A BNITM research team has provided new insights into which proteins are involved and how they work together. The researchers have published their findings in the scientific journal PLOS Biology.
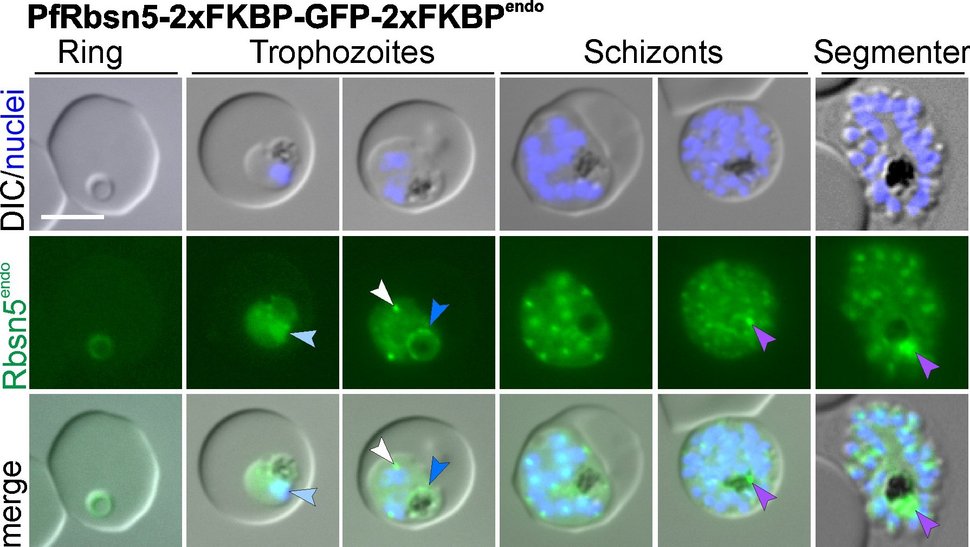
Malaria parasites hijack human red blood cells, develop in them and grow. In order to gain space and food, they ‘eat’ almost all the contents of their host cell and digest them in their food vacuole. This is mainly the protein haemoglobin. How do haemoglobin uptake and transport take place at the molecular level, which proteins are involved and where can the parasites be interfered with? The ‘Malaria Cell Biology research group’ headed by Dr Tobias Spielmann is investigating these questions.
They have now identified two proteins, Rabenosyn-5 and Rab5b, which appear to be essential for this process. During a study, they inactivated these proteins in Plasmodium falciparum parasites and found that haemoglobin-filled vesicles accumulated in their mutants and no new haemoglobin reached the digestive vacuole. The parasites could not grow any further and died. Rabenosyn-5 and Rab5b proved to be key proteins in the transport of haemoglobin to the digestive vacuole of the malaria parasite.
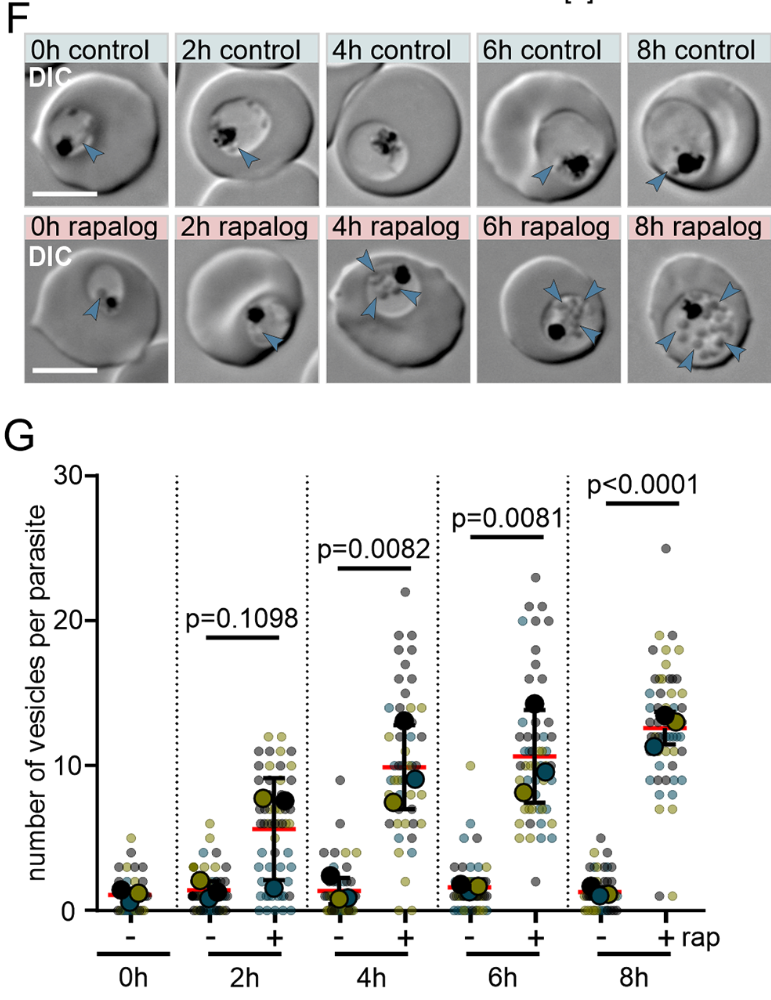
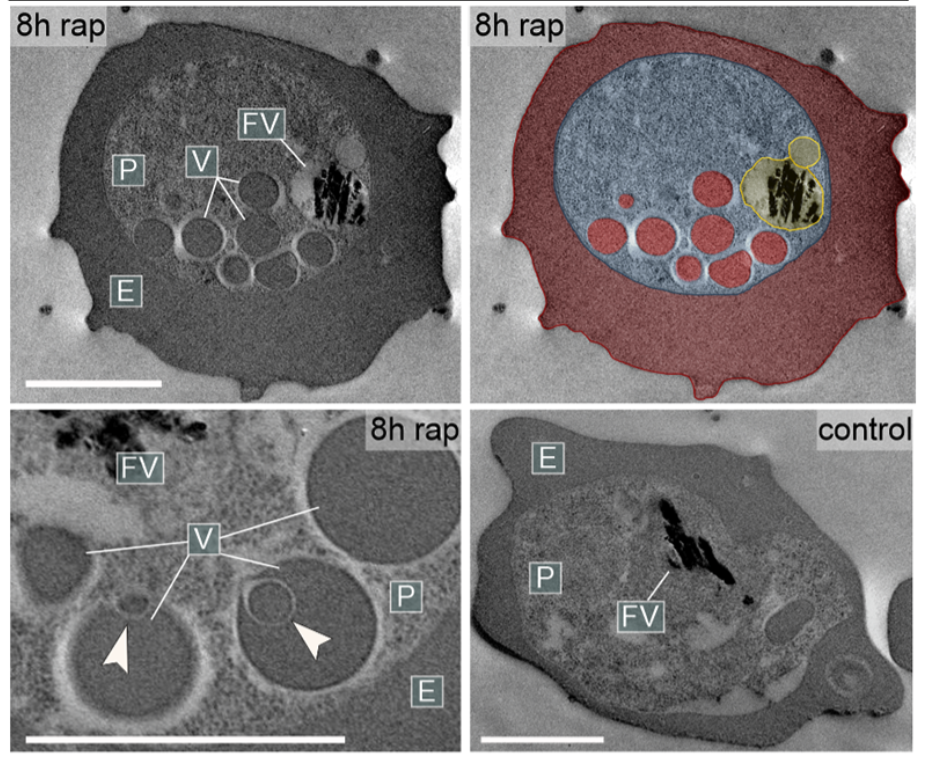
The team led by first author Ricarda Sabitzki was also able to show that these two proteins co-operate with another protein, VPS45: Together they mediate the transport of nutrients from the host cell into the digestive vacuole of the parasite.
Surprisingly, this shows that the pathogen uses similar proteins to transport ingested material as other organisms, such as humans. Only recently, the research group had shown in other studies that the actual step of taking up haemoglobin from the host cell into the cell is very unusual compared to other organisms (PMID: 38039338; PMID: 31896710); in other words, that this is a very parasite-specific uptake process.
In contrast, the team has now been able to show: This uptake process leads to a transport pathway that contains elements typical for all organisms. It can be assumed that drug development against the first part is more promising, as specific inhibition of parasite processes is easier to achieve.
“We have uncovered two crucial molecular players that help the malaria parasite to transport haemoglobin stolen from the host cell to the digestive vacuole,” says Dr Tobias Spielmann, research group leader and last author of the study. “And overall, we have gained insights into the fundamental biology of Plasmodium falciparum parasites in the blood stage. This can help to find new starting points for drug development against malaria.”
The development of new therapies against malaria is urgently needed. The most important active ingredient currently available, artemisinin, is increasingly losing its effectiveness because the parasites are developing resistance.

Background
Malaria remains one of the deadliest infectious diseases. The World Health Organisation (WHO) estimates that more than 600,000 people died worldwide in 2022 alone. Around 450,000 of these were children under the age of five. Countries south of the Sahara are the worst affected.
The study has been published in the scientific journal PLOS Biology. It was funded by the European Research Council (ERC Advanced Grant) and the German Research Foundation (DFG Graduate School 2771). Further sponsors: Jürgen Manchot Foundation and European Molecular Biology Organisation (EMBO).
Original publication: Sabitzki, R. et al.: Role of Rabenosyn-5 and Rab5b in host cell cytosol uptake reveals conservation of endosomal transport in malaria parasites. PLOS Biology May 31, 2024. DOI: 10.1371/journal.pbio.3002639
Related articels
Artemisinin-Resistance in Malaria parasites decoded: Publication in Science
European Research Council is funding cutting-edge research on drug resistance in malaria parasites: Tobias Spielmann receives ERC Advanced Grant.
Contact person
Dr Tobias Spielmann
Research group leader
Phone : +49 40 285380-486
Email : spielmann@bnitm.de
Julia Rauner
Public Relations
Phone : +49 40 285380-264
Email : presse@bnitm.de
Further information






