Understanding malaria: BNITM researchers develop innovative method
Red blood cells infected with the malaria parasite attach themselves to blood vessels. The more this happens, the more complications malaria causes. The parasite protein PfEMP1 is known to be responsible for this. Scientists at the Bernhard Nocht Institute for Tropical Medicine (BNITM) have now developed an innovative system to better analyse PfEMP1. The researchers hope that this will lead to a better understanding of malaria and, in the long term, to new therapeutic approaches. The results have recently been published as reviewed pre-print in the journal eLife.
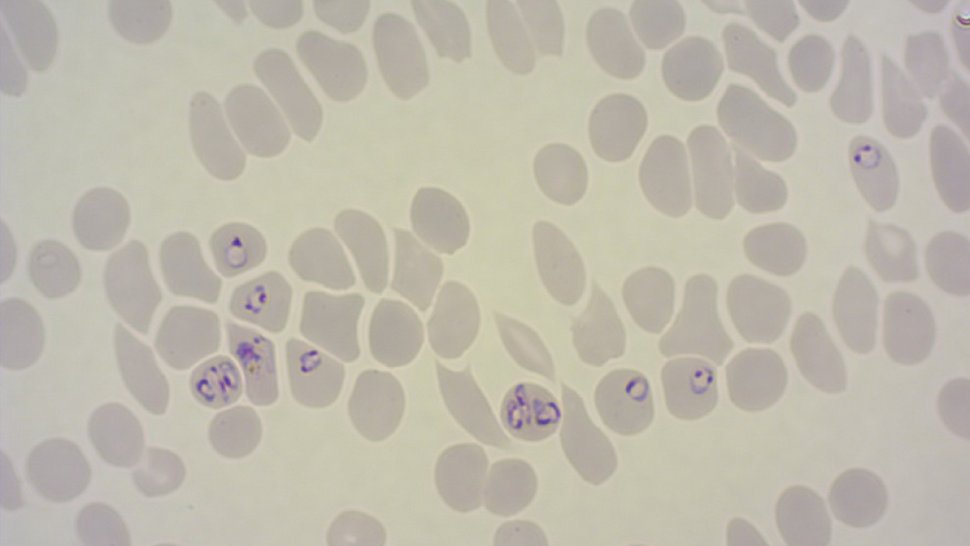
The malaria parasite Plasmodium falciparum produces the protein 'Plasmodium falciparum erythrocyte membrane protein 1' (PfEMP1) in great diversity. Each parasite encodes about 60 different variants of PfEMP1 in its genetic information. However, it only produces one variant of the protein at a time. During an infection, the parasites switch between the variants in order to evade the immune system. The diversity of PfEMP1 variants makes it difficult for researchers to analyse individual variants: In cell cultures, a population of malaria parasites produces not one, but several variants simultaneously. This makes it difficult to study the properties of individual variants.
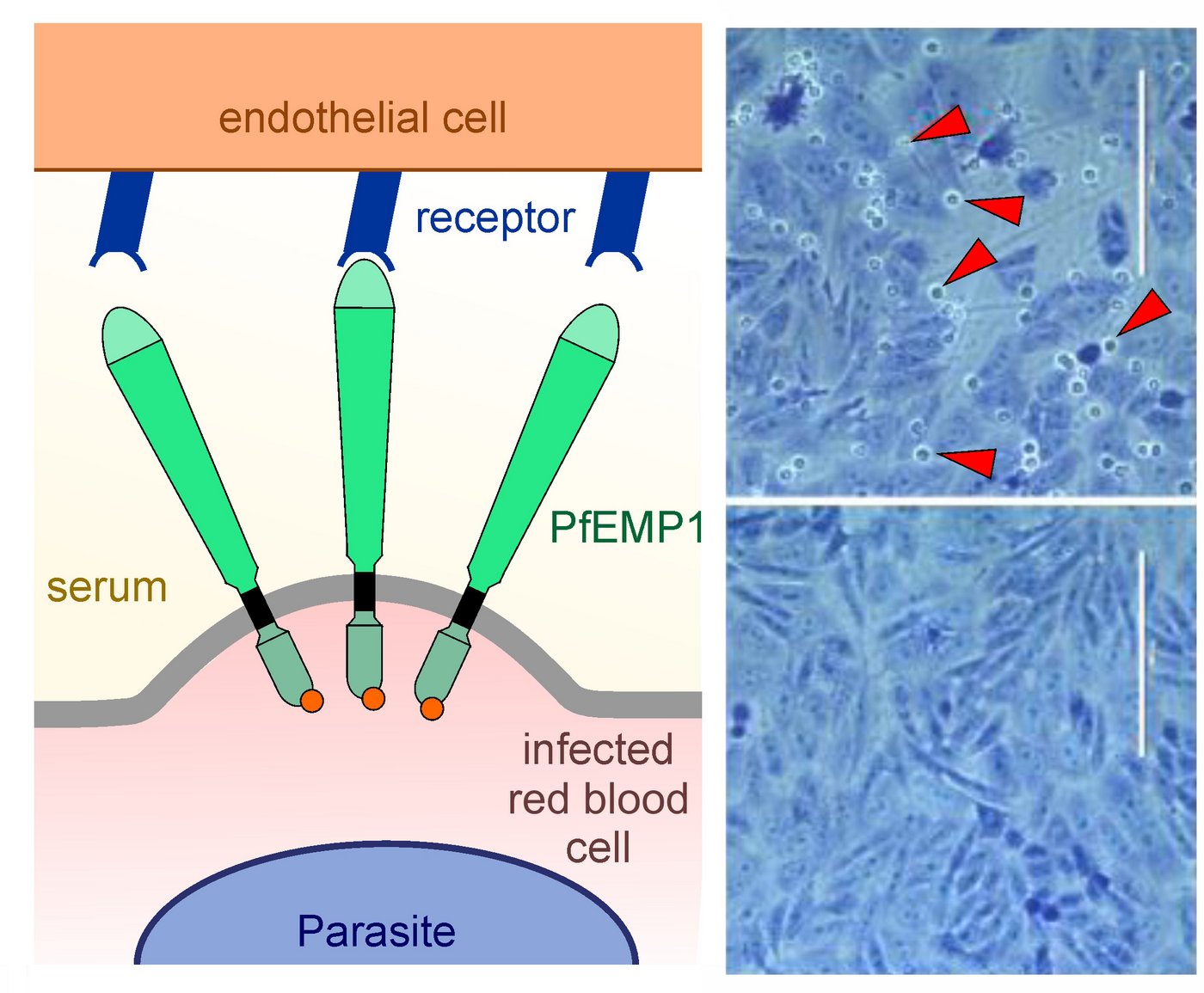
Using a method called 'Selection Linked Integration' (SLI) previously developed in the research group (Birnbaum et al., 2017 NatMethods), the researchers succeeded in generating parasite populations that exclusively produce a specific PfEMP1 variant.
“Among other things, this allows us to gain more precise insights into the role of PfEMP1 in cytoadhesion, i.e. the adhesion of infected cells to the inner wall of blood vessels,” explains Jakob Cronshagen, first author of the study.
New method can answer a wide range of questions
The SLI system can be used effectively to analyse key aspects of PfEMP1 function. For example, the researchers analysed the transport pathways of the PfEMP1 protein within infected red blood cells. They also characterised the binding properties of different PfEMP1 variants to human receptors. An advanced version of the SLI method, SLI2, allowed the research team to make additional genetic modifications in already modified parasites. Using this approach, the researchers identified two previously unknown proteins involved in the cytoadhesion process.
“Our results open up new possibilities for deciphering the mechanism of malaria pathogenesis,” explains Dr Tobias Spielmann, head of the Malaria Cell Biology research group at the BNITM. “In the long term, the knowledge gained could lead to the development of new therapeutic approaches that specifically prevent parasite cytoadhesion.”
Further Information
Original publication: Cronshagen J. et al. “A system for functional studies of the major virulence factor of malaria parasites.” eLife 2024
Publication related to the article: Birnbaum J.et al. “A genetic system to study Plasmodium falciparum protein function.” Nat Methods 2017
Contact person
Dr Tobias Spielmann
Research group leader
Phone : +49 40 285380-486
Email : spielmann@bnitm.de
Dr Anna Hein
Public Relations
Phone : +49 40 285380-269
Email : presse@bnitm.de






