Our research projects
(i) mechanisms of protective anti-helminth immunity
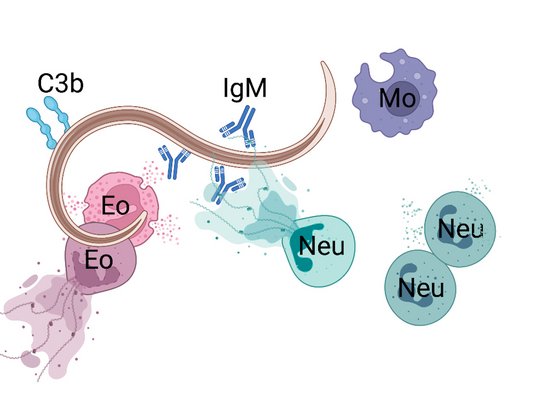
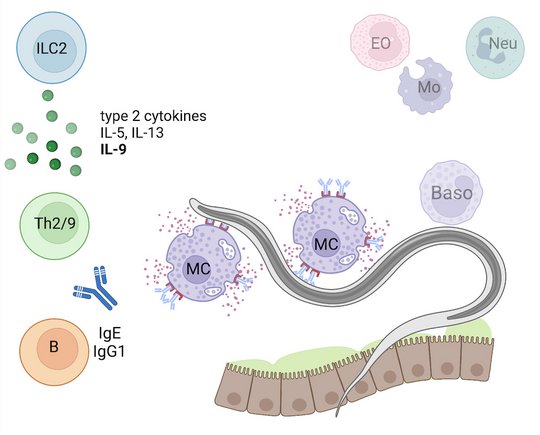
Employing novel mouse models for immune cell and receptor deficiencies we characterized the initiation and execution of anti-helminth immune responses to the parasitic helminths S. ratti and L. sigmodontis in the mouse system. We have shown that basophils and mast cells were dispensable for the control of L. sigmodontis infections in the thoracic cavity (Hartmann 2018 and Linnemann 2020) while basophils and mast cells contributed to eradication of S. ratti from the intestine (Reitz 2018a and 2016). Especially IL-9-activated mast cells were indispensable for timely termination of S. ratti infection (Reitz 2018b and 2016). Interestingly, neither IL-9 nor mast cells or basophils were needed for establishment of adaptive immune memory. They rather represented essential effectors of the early innate immune response (Breloer and Abraham 2017). This early immune response leading to the ejection of S. ratti from the intestine was triggered by release of the tissue-derived Alarmin IL-33 and strictly dependent on the presence of group 2 innate lymphoid cells (ILC2), IL-9, and mast cells, while adaptive immunity was not involved (Meiners 2020). Finally, we observed that the majority of helminth parasites were already intercepted and killed during the tissue migration by eosinophilic and neutrophilic granulocytes in the context of extracellular DNA Trap Formation (Ehrens 2021) reviewed in (Breloer and Linnemann 2024). Ongoing research addresses the exact role of ILC2 in innate and adaptive anti- S. ratti immunity and the regulation of ILC2 activation via checkpoint receptors.
(ii) mechanisms of helminth-induced immunomodulation
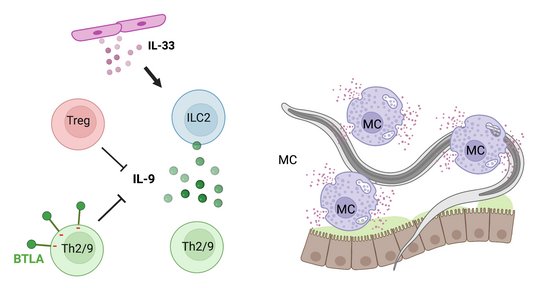
Immune competent mice eject the Strongyloides ratti after one month. Still, S. ratti is a successful parasite, as this limited time span is fully sufficient to reproduce, complete its life cycle and propagate its genes. To ensure survival for this one month, S. ratti, like most helminths, actively suppresses the immune response that is directed towards itself. We have shown that immune evasion is conveyed, among other pathways, by the expansion of Foxp3+ regulatory T lymphocytes (Treg) that antagonize IL-9 production and subsequent IL-9-mediated mast cell activation (Blankenhaus 2014 and 2011). This pathway was dominant in BALB/c mice where Treg depletion accelerated mast cell activation and reduced intestinal parasite burden. Interestingly, additional layers of regulation occurred in C57BL/6 mice that displayed a similar expansion of Treg but remained susceptible for S. ratti after Treg depletion. Next to Tregs that remain the primary regulatory pathway for maintaining peripheral tolerance, a complex network of co-inhibitory receptors acts as a second fine-tuning level of regulation. Infection with S. ratti increased expression of several co-inhibitory receptors on T cells in BALB/c and C57BL/6 mice. Of note, this induction was more pronounced in C57BL/6 mice (Hartmann 2021). Focusing on the co-inhibitory receptor B and T lymphocyte Attenuator (BTLA), we reported that either deficiency for BTLA or its ligand HVEM promoted the mast cell and IL-9-mediated anti-S. ratti immunity in C57BL/6 mice (Breloer 2015), reviewed in (Breloer and Linnemann 2024). Moreover, we described that S. ratti-derived ligands for the C type lectin receptor MINCLE antagonize eosinophil function and thus interfere with eosinophil-mediated parasite ejection from the intestine (Linnemann 2025).
(iii) impact of concurrent helminth infections on the outcome of a vaccination against different pathogens
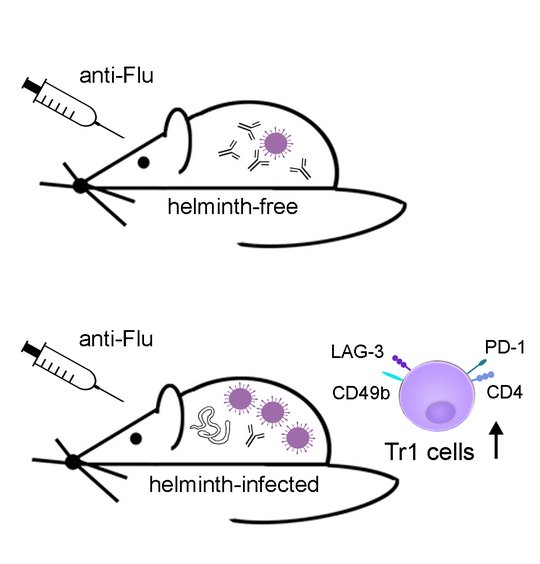
Analysing the impact of helminth-induced immunomodulation on the immune response to third party antigens we observed reduced antibody responses to model antigen immunization in mice with pre-existing L. sigmodontis infection (Hartmann 2011, 2012 and 2015; Haben 2014). Extending this observation to a clinically relevant system, we report that L. sigmodontis-infected mice that were vaccinated against seasonal influenza, using licensed human vaccines, displayed suppressed antibody responses. Protection against subsequent challenge infections with the human pathogenic 2009 pandemic H1N1 influenza A virus (swine flu) was also impaired (Hartmann 2019). Interestingly, vaccination efficacy was impaired in mice that were vaccinated during acute L. sigmodontis infection, but also in mice that were vaccinated after immune-driven resolution of L. sigmodontis infection. The suppressed status of acutely and formerly L. sigmodontis-infected mice was correlated to a sustained and systemic expansion of CD4+CD49+LAG3+IL-10+ Tr1 cells and partially abrogated by IL-10 receptor neutralization. Neither repeated vaccinations nor introduction of mild adjuvants, that were licensed for humans, restored vaccination efficacy in L. sigmodontis-infected mice (Hartmann 2022). Also drug-induced deworming did not restore the responsiveness to a vaccination given 2 weeks after treatment, as Tr1 frequencies were still high although all worms were killed at the time point of vaccination. Only a combination of deworming and improved vaccination regimen restored vaccination-induced protection against an influenza challenge infection in formerly helminth-infected mice (Stetter 2021), reviewed in (Breloer 2023).



![[Translate to English:] [Translate to English:] Logo Joachim Herz Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Joachim_Herz_Stiftung.jpg)
![[Translate to English:] [Translate to English:] Logo DFG](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DFG.png)
![[Translate to English:] [Translate to English:] Logo LCI](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/LCI_Logo_JPG.jpg)
![[Translate to English:] [Translate to English:] Logo_Alexander_von_Humboldt_Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Alexander_von_Humboldt_Stiftung.png)
![[Translate to English:] [Translate to English:] Logo of Manchot](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/manchot.jpg)





