Control or co-existence?
Surprising research results on the main vector of Lassa virus in West Africa
The Mastomys natalensis mouse is the main vector of Lassa virus. A study by the Zoonoses Control Working Group casts doubt on whether its annual control is suitable for containing the spread of Lassa fever. The results have been published in the scientific journal ‘Emerging Microbes & Infections’.

Nomen est omen: The multimammate mouse can give birth and suckle around 20 young, sometimes every 28 days. Mastomys natalensis females have two rows of up to twelve nipples on their underside - a record for mice and a problem for humans in many regions of West Africa. The nocturnal Mastomys not only destroy harvests and rob villagers of their sleep. It can also transmit the Lassa virus (LASV).
This arena virus triggers Lassa fever. The disease usually has no or only mild symptoms. However, pregnant women, babies and small children in particular can suffer haemorrhagic fever with severe internal bleeding and inflammation of the brain. If infection occurs immediately before or after birth, the death rate for infants is around 80 per cent.
In many West African countries, the virus is endemic, i.e. firmly established. According to estimates by the World Health Organisation (WHO), around 100,000-300,000 people worldwide fall ill every year, 5,000 die as a result of the infection (one to two percent).
Lassa viruses: Arena viruses of the highest biological safety level 4
There is no causal therapy for the treatment of Lassa fever. It usually consists of supportive measures to alleviate the symptoms and prevent complications. These include the administration of fluids, medication against fever and pain as well as oxygen. The antiviral drug ribavirin is only effective if it is administered at an early stage.
Vaccines are being developed, but no authorised vaccine is yet available. Lassa fever control is therefore still largely rodent control, traditionally using mouse traps and rodenticides. The idea behind this: If it is possible to keep the mouse population low, it will also be possible to contain the spread of Lassa virus.
This was also the working hypothesis of a large-scale study led by ecologist Dr Elisabeth Fichet-Calvet. She now heads the Zoonosis Control research group in the Implementation Research Section at the BNITM. For seven years, the team experimented in six villages in a rural region of Upper Guinea in West Africa. With an annual rodent control programme, they succeeded in reducing the population by up to 80 percent each time. The Lassa virus load in the mice also decreased, but only by five per cent. This was disproportionate to the effort involved. In addition, the mice reproduced extremely fast afterwards. Their numbers quickly reached the level before elimination. Did the prevalence of LASV then also increase again to the same extent? So is regular, for example annual, rodent control not sustainable at all? Mathematical modelling simulations suggested this.
Lassa fever control: so far mainly rodent control

They also came to the conclusion that continuous density control could be a more efficient means of eliminating LASV in the villages. For example, they found that LASV is eradicated within four years if the rodent population is permanently reduced by 60 per cent.
The researchers wanted to know more about this. In one of the villages, Brissa, they carried out an intensive trapping experiment: a combination of chemical control and setting up traps. They distributed rodenticides once a year for ten to 30 days. During the last year, they also intensively set snap traps in almost every house for three months. They caught rodents before and after to determine the changes in trapping success. They also analysed the animals for Lassa virus RNA and IgG antibodies.
Both measures together reduced the rodent population by 74 to 92 per cent. However, as expected, it rose again sharply shortly after the culling: After just six months, it had reached its original density. However, there was a big surprise in the infection rate of the mice: as expected, the rodenticides reduced the seroprevalence by five per cent. However, the intensive trapping actually led to a significantly higher infection rate: the seroprevalence increased from 28 to 67 per cent.
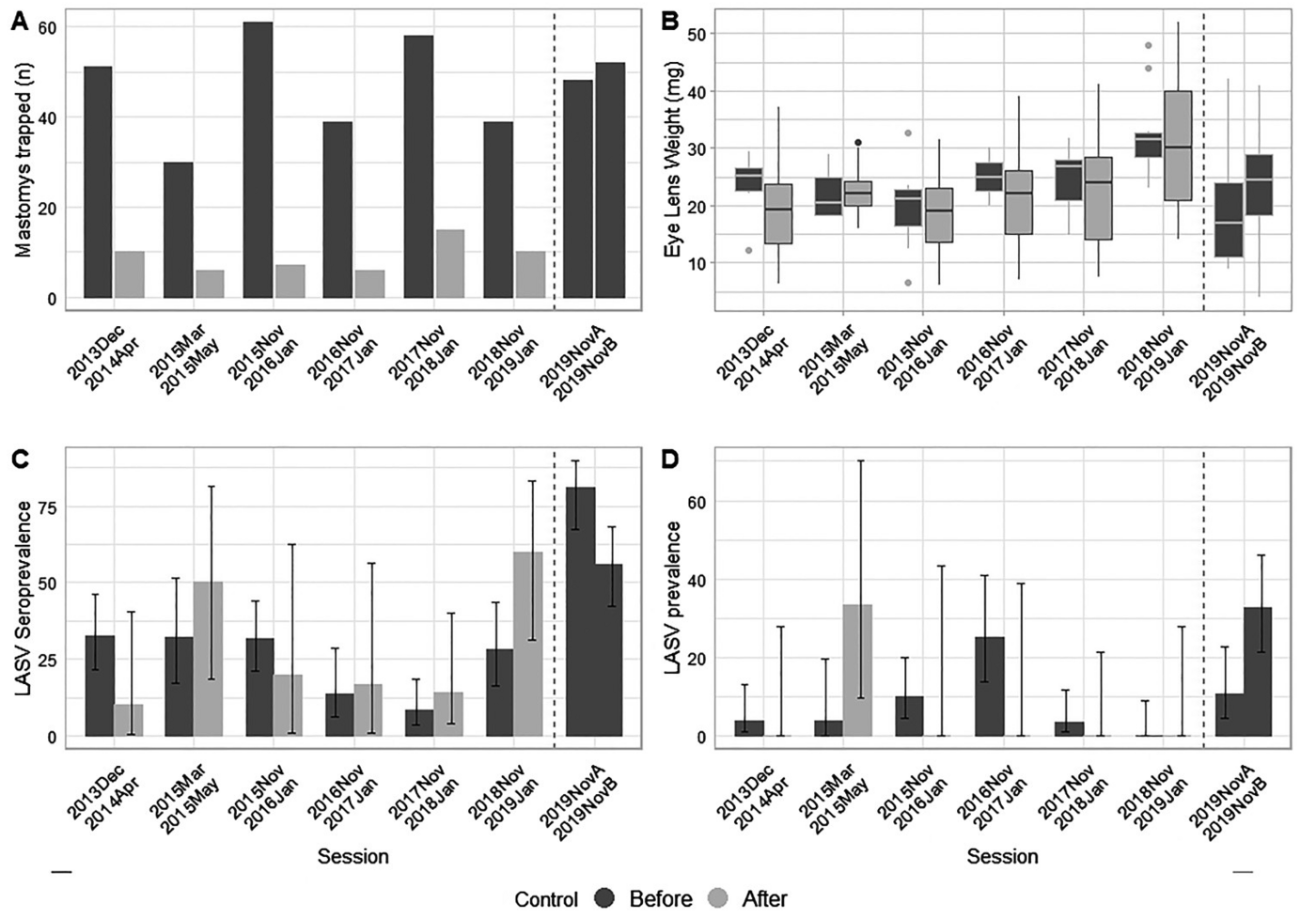
Fig. on the right: Infection rate of Lassa virus in the rodent population in Brissa, derived from age-specific seroprevalence data. Left: LASV seroprevalence depicted in function of eye lens weight for trapping sessions 1–6 (2013–2018). No difference in the infection rate was found when comparing trapping sessions before (black) and after chemical rodent control (grey). Right: Significant differences were found in the infection rate between years. Solid lines represent estimates derived from a generalized linear model with 95% confidence intervals (dotted lines).
Fig. on the left: A) Number of Mastomys natalensis captured per 3-night trapping session in Brissa, both before and after rodenticide treatment. B) Boxplots illustrating the distribution of eye lens weights in captured Mastomys natalensis (a known proxy for age in mammals), categorized by trapping session before and after rodenticide treatment. C) Lassa seroprevalence (IgG) during trapping session, both before and after rodenticide treatment. D) Lassa prevalence (PCR) by session, both before and after rodenticide treatment. In dark grey the sessions before rodenticide treatment and light grey sessions after rodenticide treatment. The dotted lines signify the intensive snap-trapping session that took place between 17/02/2019 and 15/05/2019.

The team of authors explains these results on the one hand with the fertility of the multimammate mice described above: Apparently they were able to compensate for the decline in their population density extremely quickly, especially after chemical control. This could be related to the fact that older animals have their favourite food sources and therefore ingested fewer chemical baits: The population was ageing, the surviving animals were already capable of reproducing and quickly compensated for the considerable losses in the younger generation. At the same time, they were already chronically Lassa-infected: the seroprevalence increased.

The research team therefore concludes that the annual chemical control of Mastomys natalensis alone or together with traps is ineffective or even counterproductive.
The head of the study and last author of the paper Dr Elisabeth Fichet-Calvet: "After so many years of intensive experimentation and observation, we have considerable doubts as to whether rodent control is at all suitable for combating Lassa fever. In the areas where we have conducted our research, the virus circulates to such an extent that the population's immunity is very high and severe cases are rare." The situation is different in cities, where the population is more mobile and the virus is more likely to affect people who are not immune.
Overall, it could be more sensible and also more cost-effective to set snap traps all year round and/or lay out baits with hormones to control the fertility of the rodents.
Original publication
Mariën, J. et al. (2024). Rodent control strategies and Lassa virus: some unexpected effects in Guinea, West Africa. Emerging Microbes & Infections, 13(1). https://doi.org/10.1080/22221751.2024.2341141
About the Bernhard Nocht Institute for Tropical Medicine (BNITM)
The Bernhard Nocht Institute for Tropical Medicine (BNITM) is Germany's largest institution for research, care and teaching in the field of tropical and emerging infectious diseases. BNITM research has always focussed on global health / One Health and on translation - the transfer of basic research into application. This research approach is also reflected in the Institute's five sections: Pathogen (pathogen) -> Interface (immunology, host/pathogen) -> Patient (clinic) -> Population (epidemiology) -> Implementation (successful application and establishment of knowledge).
Current thematic priorities are malaria, haemorrhagic fever viruses, neglected tropical diseases (NTDs), immunology, epidemiology and the clinic of tropical infections as well as the mechanisms of virus transmission by mosquitoes. For the handling of highly pathogenic viruses and infected insects, the institute has laboratories of the highest biological safety level (BSL4) and a safety insectarium (BSL3). The BNITM's mobile laboratories are available for global outbreak control of highly pathogenic or highly infectious viruses.
The BNITM is a National Reference Centre for the detection of all tropical infectious agents, a WHO Collaborating Centre for Arboviruses and Haemorrhagic Fever Viruses, a WHO Collaborating Centre for Behavioural Research for Global Health and an institute in the Leibniz Association.
Together with the Ghanaian Ministry of Health and the University of Kumasi, the BNITM operates a modern research and training centre in the West African rainforest, which is also available to external working groups. The institute also maintains numerous other collaborations in other African countries such as Gabon, Nigeria, Tanzania and Madagascar.
Contact person
PhD Elisabeth Fichet-Calvet
Research Group Leader
Phone : +49 40 285380-942
Email : fichet-calvet@bnitm.de
Julia Rauner
Public Relations
Phone : +49 40 285380-264
Email : presse@bnitm.de
Further information






