Our research projects at a glance
Despite considerable efforts during recent years to combat malaria, the disease remains a major threat to public health in tropical countries. The most severe clinical courses of malaria are due to infections with the protozoan species Plasmodium falciparum. The virulence of P. falciparum has been linked to the ability of infected red blood cells to adhere to a range of endothelial cell surface molecules expressed on blood vessel walls such as CD36, EPCR and ICAM-1. This so-called sequestration process allows the parasite to avoid spleen-dependent killing mechanisms. However, membrane proteins mediating sequestration are exposed to the host’s immune system and need to be varied in a long-lasting chronic infection by undergoing antigenic variation. For this reason, P. falciparum possesses several multicopy gene families coding for variant surface antigens, which are characterized by an extensive sequence polymorphism. The best known is the high molecular weight PfEMP1 family (P. falciparum erythrocyte membrane protein 1), which is encoded by 60 vargenes per parasite genome. Each var gene encodes a similar, but not identical PfEMP1 version with particular receptor binding capacities. The expression switching between different var genes enables the parasite to change its immunological appearance and cytoadhesion capability during the course of infection. Accordingly, blocking the expression switch between different PfEMP1 variants exposed on the surface of infected erythrocytes would give the immune system a major advantage in fighting malaria infections. Additionally, disease-causing PfEMP1 variants or other virulence factors could be used for an anti-disease intervention.
Var gene expression in experimental human malaria infections
How the parasite coordinates its var gene expression in vivo is of tremendous interest and we are currently taking advantage of controlled human malaria infection (CHMI) studies to address this question. During CHMI studies either malaria-naïve or pre-exposed volunteers are infected with one lot of purified, cryopreserved sporozoites, the infectious mosquito stage of the parasite, provided by Sanaria Inc., USA. Parasite-positive blood from the volunteers is immediately preserved for transcript profiling of the entire var gene repertoire either by quantitative real time PCR (qPCR) or by RNA sequencing. For comparison, in vitro cultivated ‘pre-mosquito parasites’, gametocytes and sporozoites are also analysed. We aim to compare var gene expression patterns i) between different parasite stages, ii) between in vitro-cultivated parasites and those obtained from the volunteers, iii) between parasites recovered from malaria-naïve and semi-immune volunteers, iv) between in vivo samples obtained longitudinally during the course of volunteer infections, and v) between the genetically different and geographically distant parasite strain. These analyses allow us to uncover parasites gene expression at the early onset of P. falciparum blood infections in malaria-naïve and semi-immune individuals, to determine how this expression pattern is modulated by the host immunity during the course of infection and how the expression might be controlled.
Key publications:
Wichers-Misterek et al. 2023 PLoS Pathogens; DOI: 10.1371/journal.ppat.1011468.
Bachmann et al. 2019 PLoS Pathogens; DOI: 10.1371/journal.ppat.1007906.
Bachmann et al. 2016 PLoS Pathogens; DOI: 10.1371/journal.ppat.1005538.
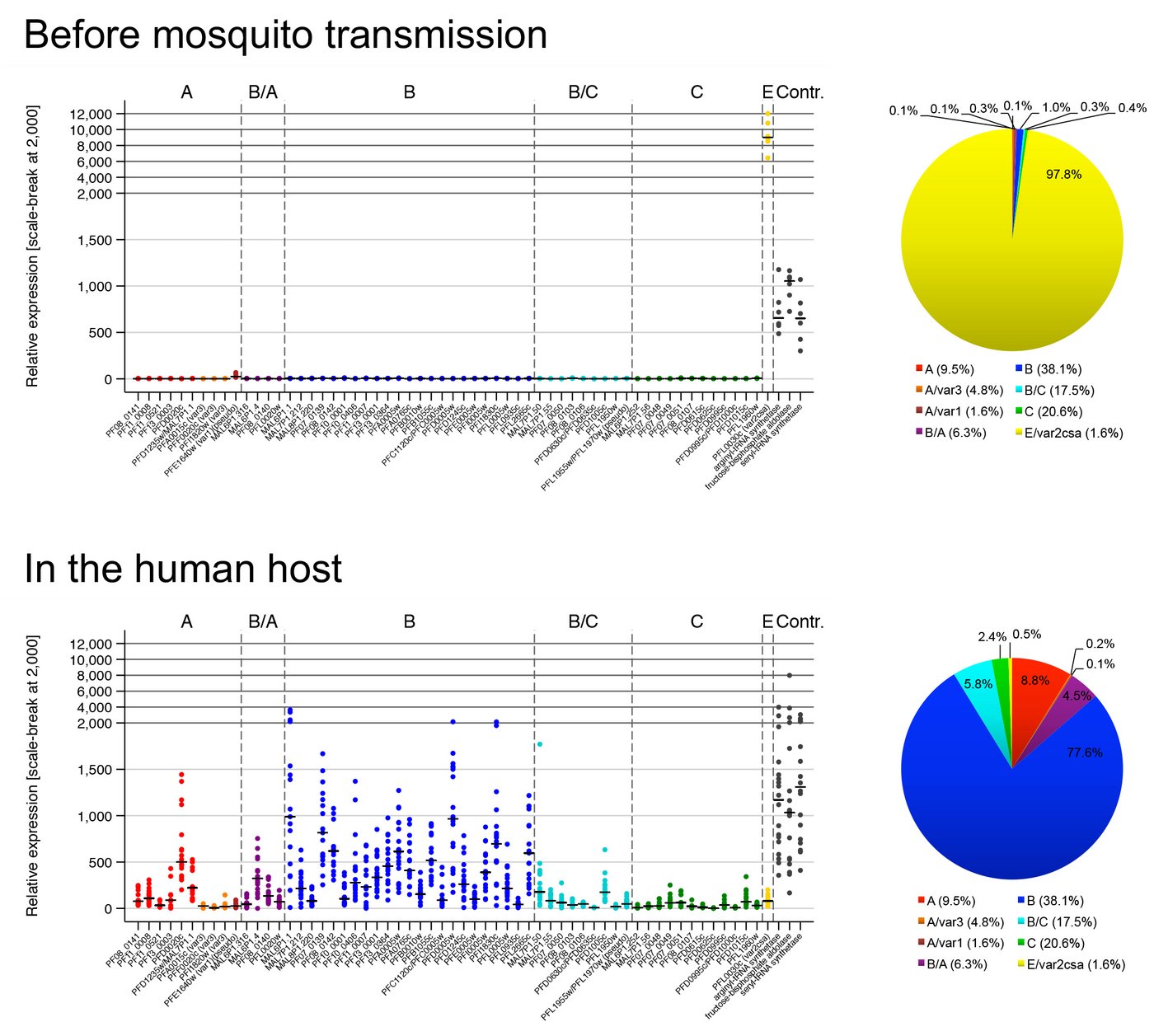
©Anna Bachmann
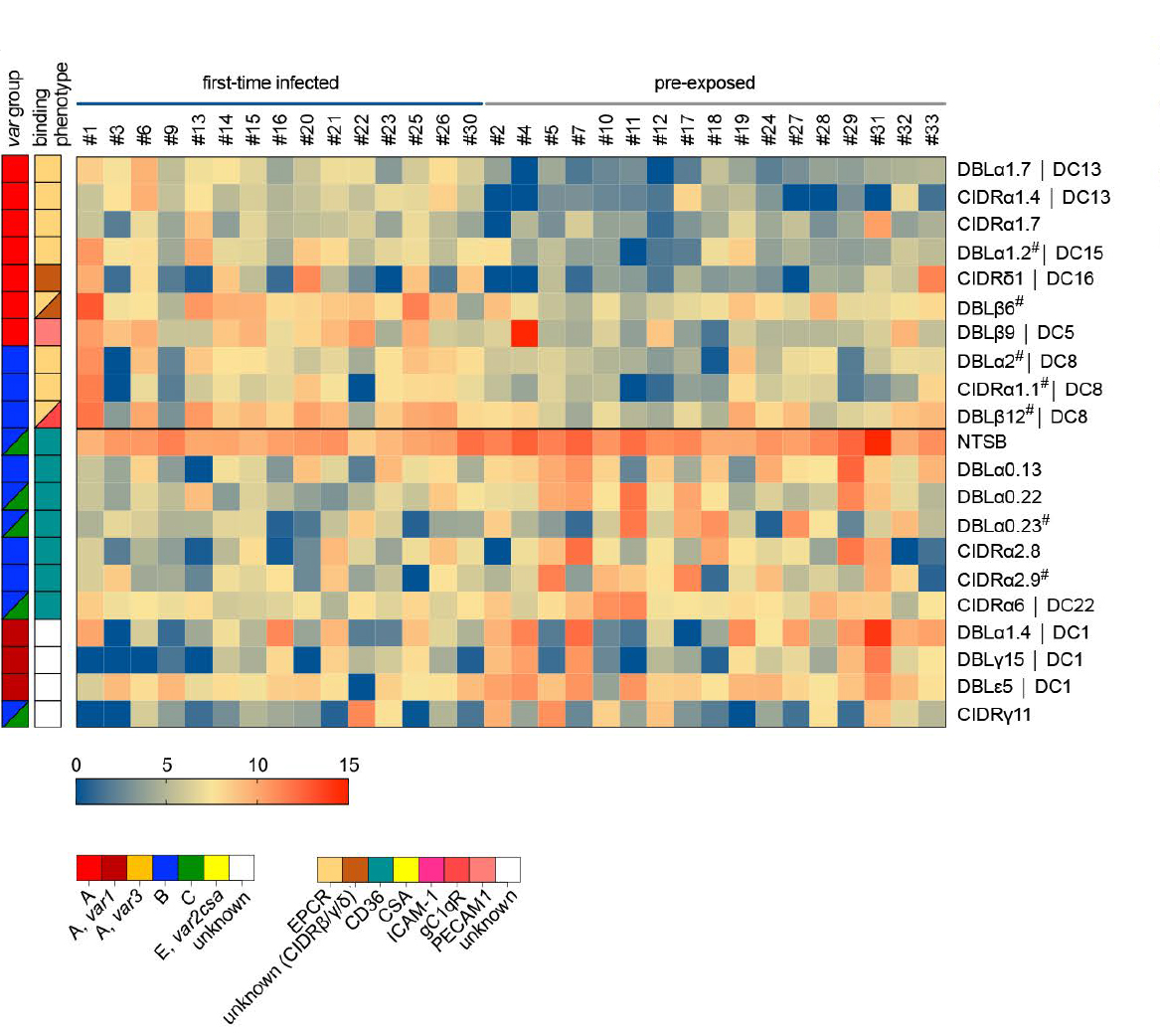
Virulence-associated gene expression patterns in natural human malaria infections
Mainly children under five years of age and pregnant women from malaria-endemic regions suffer from severe malaria, but adults from areas of lower endemicity and non-immune travelers are also vulnerable to severe disease. Although severe malaria manifests as three main overlapping syndromes in children (cerebral malaria, hyperlactatemia/acidosis and severe anemia), disease symptomatology also varies according to age with more multiorgan complications and a higher fatality rate in adults. The underlying factors responsible for the different disease syndromes in children or age-related differences seen in adults remain largely unknown. To better understand the biological processes involved in host-pathogen interactions we are analysing samples i) from a Ghanaian cohort of paediatric malaria patients with accurately defined phenotypes of the different severe malaria syndromes and asymptomatic controls or ii) from returning adult travellers with varying previous malaria exposure and different clinical manifestations. We use an mRNA-sequencing and analysis pipeline to uncover associations of parasites gene expression (including var genes) with the different malaria syndromes, disease severity and a naïve immune status.
Key publications:
Andradi-Brown et al. 2023 eLife (reviewed preprint); DOI: 10.7554/elife.87726.1.
Wichers et al. 2021 eLife; DOI: 10.7554/elife.69040.
Gene expression, subcellular localization and function of small variant surface antigens
In addition to PfEMP1, which certainly mediates antigenic variation and endothelial receptor binding of P. falciparum, much less is known about the small variant surface antigens families RIFIN, STEVOR and PfMC-2TM. Interestingly, they members have been localized at different subcellular sites within the infected red blood cell and are, therefore, candidates for mediating several of the patho-physiological events during malaria disease. However, a better understanding of these small variant surface protein families is necessary and may reveal new targets for intervention. To get more insight in their function, we established analytical tools for analyzing parasites obtained from malaria patients, whose gene expression and protein localization is not influenced by prolonged in vitro-cultivation. We could already show that the gene expression of RIFIN- and STEVOR-encoding genes is enhanced in vivo and that proteins of both families contribute to the surface coat of the infected red blood cell. We are currently characterizing the gene expression, localization, interaction partners, and functions of the small surface antigens at different life cycle stages of the parasite.
Key publications:
Wichers et al. 2019 mBio; DOI: 10.1128/mbio.01500-19.
Bachmann et al. 2015 Malaria Journal; DOI: 10.1186/s12936-015-0784-2.
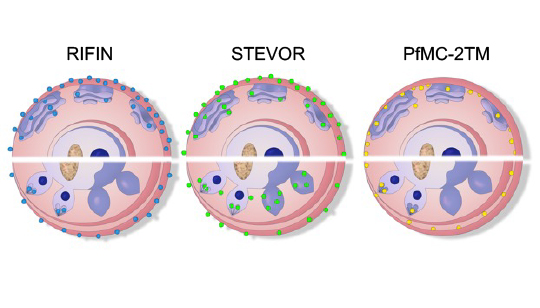
©Marthe Janßen | Anna Bachmann



![Logo Deutsches Zentrum für Infektionsforschung (DZIF) [Translate to English:] Logo des DZIF in blau auf weißem Hintergrund](/fileadmin/media/Forschung/forschungsgruppen/Pathogen/Abteilung_Zellulaere_Parasitologie/Laborgruppe_Bachmann/Deutsches_Zentrum_fuer_Infektionsforschung_logo.svg)
![[Translate to English:] [Translate to English:] Logo DFG: Zu sehen ist der Schriftzu DFG in dicker blauer Schrift.](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DFG.png)
![[Translate to English:] [Translate to English:] Das Logo Uni Hamburg: Ein rotes Quadrat mit weißer Inschrift und weißem Hamburg-Wappen.](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/UHH_Logo.jpg)





