Attacking the malaria pathogen by the digestive tract
BNITM junior research group provides important insights into the metabolism of the parasite
Researchers often find drugs without first understanding their exact conditions and mechanisms of action. At the latest when resistance threatens, however, this is precisely what is crucial. In the case of the malaria drug chloroquine, it was thought for more than half a century that the acidic environment in the parasite's digestive "organ" was a prerequisite for its effectiveness. A research group at the Bernhard Nocht Institute for Tropical Medicine (BNITM) has disproved this assumption and gained further surprising insights into the protein complex that serves to acidify the parasite's digestive "organ". The results have been published in the journal The Proceedings of the National Academy of Sciences (PNAS).
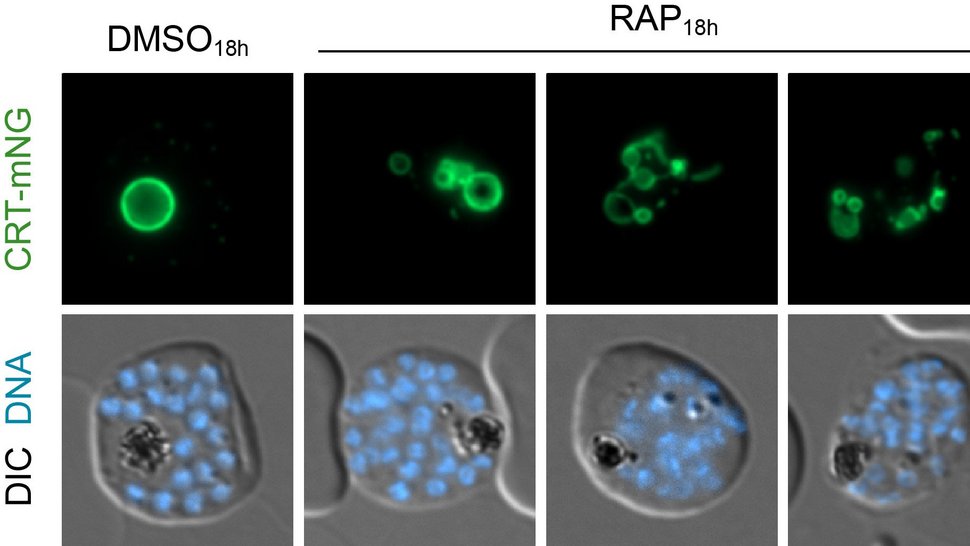
Malaria parasites hijack red blood cells, nest inside, "eat" the red blood pigment, grow and multiply until the blood cell bursts and the game begins again - the blood phase.
Many antimalarial drugs work by causing the parasite to die from its own breakdown products, which are produced when the red blood pigment is digested inside an acidified digestive vacuole. The best known example of this is chloroquine. The membrane of the digestive vacuole (digestive "organ") is a particularly important interface here. It plays a key role for the natural metabolic processes as well as for the absorption of active substances.
The BMBF junior research group "Molecular Parasitology" at the BNITM has analysed the metabolic processes at the digestive vacuole membrane in detail using various innovative methods and has been able to clarify important open questions.
For example, the so-called proton pump, the V-ATPase, is located in the membrane of the food vacuole. It continuously pumps positively charged protons from the cell sap of the parasite into its food vacuole. In this way, it ensures an acidic environment in this digestive "organ". This is necessary - not unlike in the human stomach - to be able to break down proteins efficiently.
The researchers led by Dr Joachim Michael Matz wanted to know what happens when they switch off parts of the proton pump and thus disrupt the acidification of the food vacuole. To do this, they used a combination of different molecular and cell biological methods such as CRISPR/Cas9, conditional gene deletions as well as fluorescence and electron microscopy.
Chloroquine does not only like it acidic
The working hypothesis was: If the pH value in the food vacuole of the malaria parasite increases, i.e. becomes less acidic, this weakens the parasite or even kills it. At the same time, however, less of the malaria drug chloroquine accumulates there. According to common doctrine, chloroquine binds the protons, of which far fewer were now available in the food vacuole.
The first assumption was confirmed. Surprisingly, however, the accumulation of chloroquine in the food vacuole was about the same.
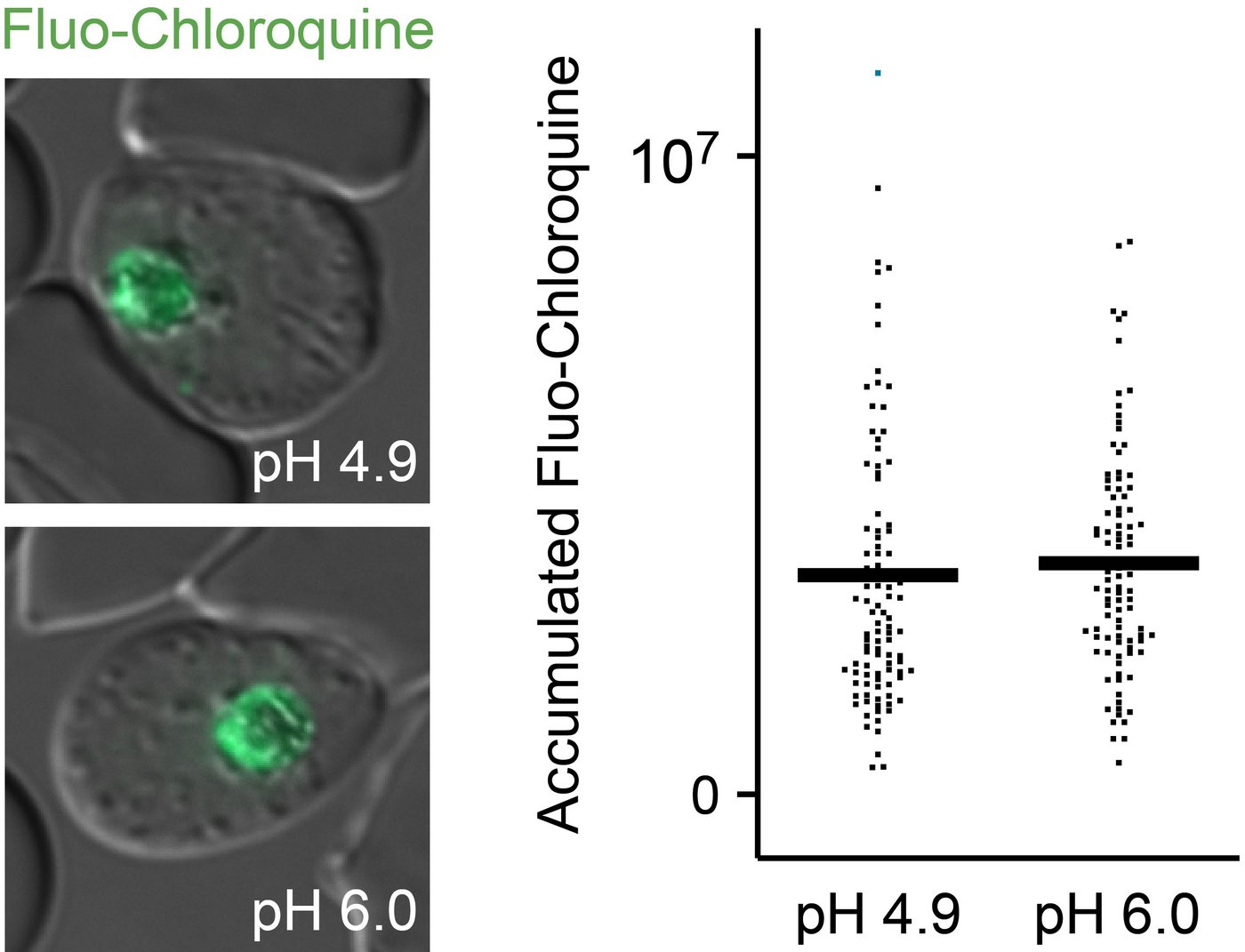
This fundamentally contradicts the previous doctrine of the absorption mechanism of chloroquine.
Until now, it was assumed that chloroquine molecules bind to the protons in the malaria parasite's digestive vacuole and, as positively charged particles, would not be membrane-permeable. This should lead to the accumulation of chloroquine in the digestive vacuole, where it inhibits the detoxification of the parasite's own degradation products.
But apparently other reasons lead to the accumulation of chloroquine in the food vacuole. Joachim Matz's research group is currently investigating which alternative transport processes could underlie this instead, e.g. parasite-own transporter proteins. This is also important for the development of novel antimalarial drugs that are supposed to work in the digestive vacuole.
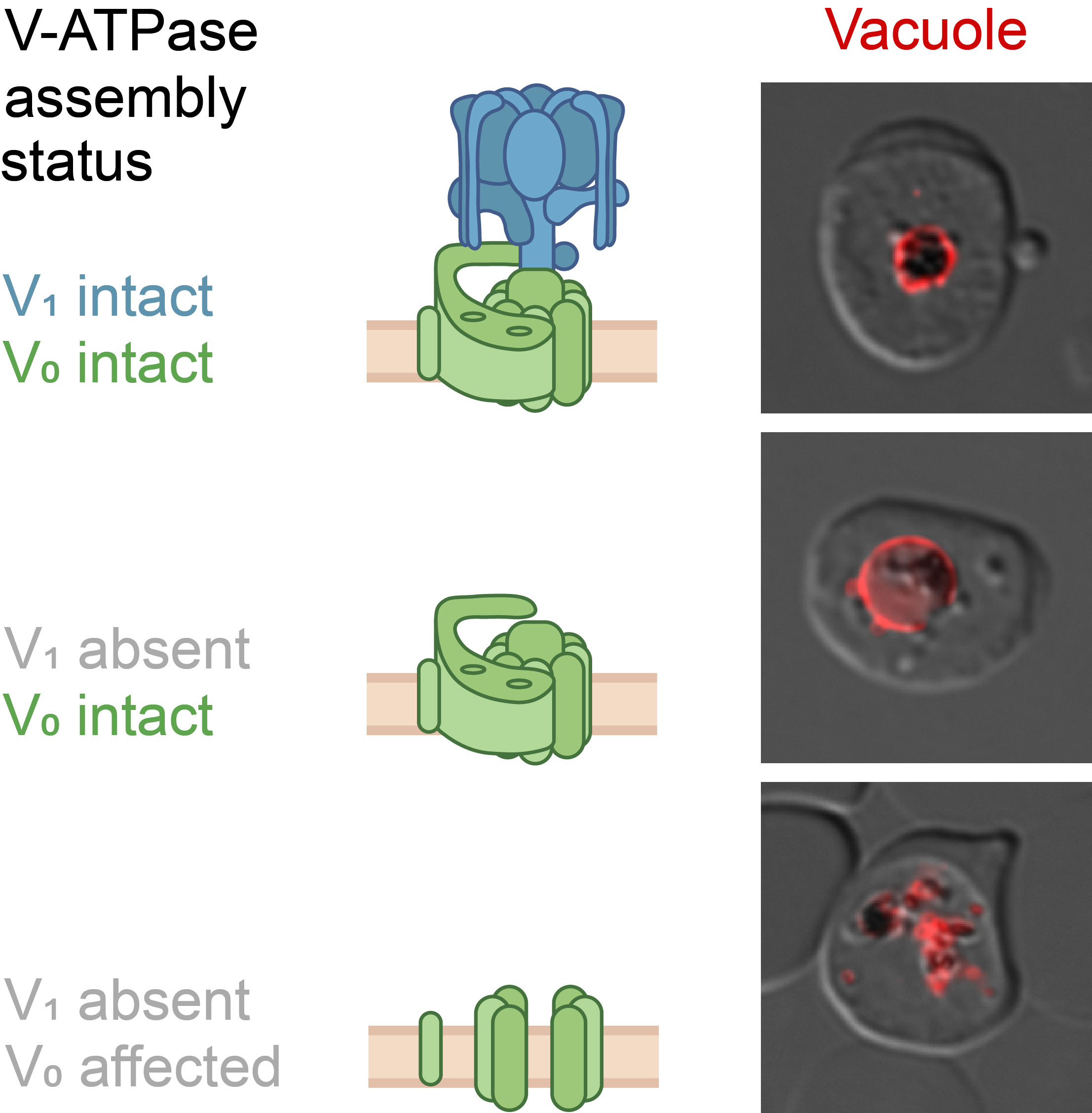
Proton pumps do not only pump protons
An interesting detail of the study: It made a big difference in the experiments whether the part of the proton pump that protrudes into the cell sap or the part that lies in the membrane of the digestive vacuole was manipulated. When the researchers switched off a protein within the part in the cell sap, the digestive vacuole inflated because the red blood pigment could no longer be broken down efficiently. But when they switched off a protein of the part in the membrane, the digestive vacuole collapsed and broke up into many individual parts. This means that this part of the proton pump apparently has the additional function of holding the parasite's digestive vacuole together.
The head of the BMBF research group, Dr Joachim Michael Matz: "The biological acidification process of the food vacuole and the molecular machinery that controls it are still largely unexplored. The results of our study provide a number of important pieces of the puzzle. Among other things, they show that the proton pump V-ATPase plays a key role in the acidification of the food vacuole and that its individual components apparently have quite different functions".
This is where the research group now wants to move on and further clarify the functions of the individual proteins of the proton pump on both sides of the membrane of the food vacuole.
Contact person
Dr Joachim Michael Matz
Research Group Leader
Phone : +49 40 285380-301
Email : joachim.matz@bnitm.de
Julia Rauner
Public Relations
Phone : +49 40 285380-264
Email : presse@bnitm.de
Further information







